IN VITRO TRANSPLANTATION OF GENETICALLY MODIFIED CELLS TO THE TENDON SURFACE
- PMID: 20209046
- PMCID: PMC2832611
- DOI: 10.1142/S0218957708001961
IN VITRO TRANSPLANTATION OF GENETICALLY MODIFIED CELLS TO THE TENDON SURFACE
Abstract
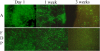
The objective of this paper was to study in vitro transfection of tendon cells and adherence of transfected cells to different tendon surfaces. Achilles tendon fibroblasts from 2-month-old New Zealand white rabbits were cultured to confluence, after which the cells were transfected by an adenovirus carrying either the β-galactosidase reporter gene or the green fluorescent protein (GFP) gene at multiplicities of infection (MOIs) of 50, 100, or 500. Two days later, the cells were transplanted onto the surfaces of rabbit Achilles, peroneus brevis, flexor profundus, and extensor longus tendons. The tendons were assessed by X-gal staining after 9 days, and by GFP fluorescence at 7, 14, and 21 days. Twenty percent to 50% of the treated cells stained for β-galactosidase at an MOI of 500. The GFP-labeled cells showed nearly 100% fluorescence at an MOI of 50. No positive cells were visible in the control group. The β-galactosidase and GFP-expressing cells remained viable for as long as 3 weeks. It is possible to introduce foreign genes into rabbit tendon cells, transplant the cells onto tendon surfaces, and maintain viability of the cell/tendon construct for several weeks.
Figures



Similar articles
-
[EFFECT OF RECOMBINANT ADENOVIRUS-BONE MORPHOGENETIC PROTEIN 12 TRANSFECTION ON DIFFERENTIATION OF PERIPHERAL BLOOD MESENCHYMAL STEM CELLS INTO TENDON/LIGAMENT CELLS].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2015 Apr;29(4):472-6. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2015. PMID: 26477162 Chinese.
-
[EFFECT OF HAMSTRING TENDON TRANSFECTED WITH ADENOVIRUS- MEDIATED TRANSFORMING GROWTH FACTOR β₁ GENE ON HISTOMORPHOLOGY OF TENDON-BONE INTERFACE HEALING AFTER ANTERIOR CRUCIATE LIGAMENT RECONSTRUCTION IN RABBITS].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2015 Dec;29(12):1488-93. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2015. PMID: 27044216 Chinese.
-
Mechanical properties of reconstructed achilles tendon with transfer of peroneus brevis or flexor hallucis longus tendon.J Foot Ankle Surg. 2007 Nov-Dec;46(6):424-8. doi: 10.1053/j.jfas.2007.07.003. J Foot Ankle Surg. 2007. PMID: 17980837
-
Catastrophic Failure of an Infected Achilles Tendon Rupture Repair Managed with Combined Flexor Hallucis Longus and Peroneus Brevis Tendon Transfer.Clin Podiatr Med Surg. 2016 Jan;33(1):153-62. doi: 10.1016/j.cpm.2015.06.006. Epub 2015 Jul 8. Clin Podiatr Med Surg. 2016. PMID: 26590732 Review.
-
Matrix metabolism and healing in the flexor tendon. Experimental studies on rabbit tendon.Scand J Plast Reconstr Surg Hand Surg Suppl. 1991;23:1-51. Scand J Plast Reconstr Surg Hand Surg Suppl. 1991. PMID: 1947826 Review.
References
-
- Anderson WF. Prospects for human gene therapy. Science. 1984;226:401–409. - PubMed
-
- Bainbridge JW, Mistry AR, Thrasher AJ, Ali RR. Gene therapy for ocular angiogenesis. Clin Sci. 2003;104:561–575. - PubMed
-
- Bonadio J. Tissue engineering via local gene delivery. J Mol Med. 2000;78:303–311. - PubMed
-
- Boyer MI, Strickland JW, Engles D, Sachar K, Leversedge FJ. Flexor tendon repair and rehabilitation: State of the art in 2002. Instr Course Lect. 2003;52:137–161. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
