Scyl1 regulates Golgi morphology
- PMID: 20209057
- PMCID: PMC2832016
- DOI: 10.1371/journal.pone.0009537
Scyl1 regulates Golgi morphology
Abstract
Background: Membrane trafficking is a defining feature of eukaryotic cells, and is essential for the maintenance of organelle homeostasis and identity. We previously identified Scy1-like 1 (Scyl1), a member of the Scy1-like family of catalytically inactive protein kinases, as a high-affinity binding partner of COPI coats. COPI-coated vesicles control Golgi to endoplasmic reticulum trafficking and we observed that disruption of Scyl1 function leads to a decrease in trafficking of the KDEL receptor via the COPI pathway. We reasoned that if Scyl1 plays a major role in COPI trafficking its disruption could influence Golgi homeostasis.
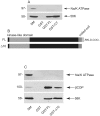
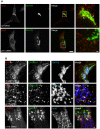
Methodology/principal findings: We performed Scyl1 knock down in cultured cells using previously established methods and observed an alteration in Golgi morphology. Both the surface area and volume of the Golgi is increased in Scyl1-depleted cells, but the continuity and polarity of the organelle is unperturbed. At the ultrastructural level we observe a decrease in the orderly structure of the Golgi with an increase in cisternal luminal width, while the number of Golgi cisternae remains unchanged. The golgin family of proteins forms a detergent resistant network that controls Golgi homeostasis. Disruption of this protein network by knock down of the golgin p115 disrupts the Golgi localization of Scyl1. Moreover, we find that Scyl1 interacts with 58K/formiminotransferase cyclodeaminase (FTCD), a protein that is tightly associated with the cis face of the Golgi.
Conclusions/significance: Our results place Scyl1 at an interface between the golgin network and COPI trafficking and demonstrate that Scyl1 is required for the maintenance of Golgi morphology. Coupled with the observation from others that Scyl1 is the gene product responsible for the neurodegenerative mouse model mdf, our results additionally implicate the regulation of COPI trafficking and Golgi homeostasis in neurodegeneration.
Conflict of interest statement
Figures
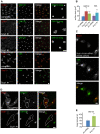
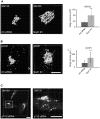


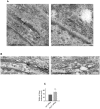
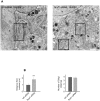

References
-
- Kirchhausen T. Three ways to make a vesicle. Nat Rev Mol Cell Biol. 2000;1:187–198. - PubMed
-
- Malsam J, Satoh A, Pelletier L, Warren G. Golgin tethers define subpopulations of COPI vesicles. Science. 2005;307:1095–1098. - PubMed
-
- Storrie B, Pepperkok R, Nilsson, T Breaking the COPI monopoly on Golgi recycling. Trends Cell Biol. 2000;10:385–391. - PubMed
-
- Short B, Haas A, Barr FA. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim Biophys Acta. 2005;1744:383–395. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

