Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice
- PMID: 20209061
- PMCID: PMC2832020
- DOI: 10.1371/journal.pone.0009539
Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice
Abstract
Background: Chronic inflammation is a characteristic feature of diabetic cutaneous wounds. We sought to delineate novel mechanisms involved in the impairment of resolution of inflammation in diabetic cutaneous wounds. At the wound-site, efficient dead cell clearance (efferocytosis) is a pre-requisite for the timely resolution of inflammation and successful healing.
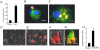
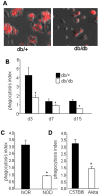
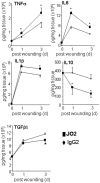
Methodology/principal findings: Macrophages isolated from wounds of diabetic mice showed significant impairment in efferocytosis. Impaired efferocytosis was associated with significantly higher burden of apoptotic cells in wound tissue as well as higher expression of pro-inflammatory and lower expression of anti-inflammatory cytokines. Observations related to apoptotic cell load at the wound site in mice were validated in the wound tissue of diabetic and non-diabetic patients. Forced Fas ligand driven elevation of apoptotic cell burden at the wound site augmented pro-inflammatory and attenuated anti-inflammatory cytokine response. Furthermore, successful efferocytosis switched wound macrophages from pro-inflammatory to an anti-inflammatory mode.
Conclusions/significance: Taken together, this study presents first evidence demonstrating that diabetic wounds suffer from dysfunctional macrophage efferocytosis resulting in increased apoptotic cell burden at the wound site. This burden, in turn, prolongs the inflammatory phase and complicates wound healing.
Conflict of interest statement
Figures




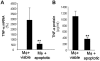
References
-
- Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–387. - PubMed
-
- Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. Journal of Leukocyte Biology. 2001;69:513–521. - PubMed
-
- Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

