The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide
- PMID: 20209079
- PMCID: PMC2831066
- DOI: 10.1371/journal.pone.0009505
The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide
Abstract
Background: The amyloid beta-protein (Abeta) is believed to be the key mediator of Alzheimer's disease (AD) pathology. Abeta is most often characterized as an incidental catabolic byproduct that lacks a normal physiological role. However, Abeta has been shown to be a specific ligand for a number of different receptors and other molecules, transported by complex trafficking pathways, modulated in response to a variety of environmental stressors, and able to induce pro-inflammatory activities.
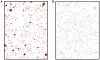
Methodology/principal findings: Here, we provide data supporting an in vivo function for Abeta as an antimicrobial peptide (AMP). Experiments used established in vitro assays to compare antimicrobial activities of Abeta and LL-37, an archetypical human AMP. Findings reveal that Abeta exerts antimicrobial activity against eight common and clinically relevant microorganisms with a potency equivalent to, and in some cases greater than, LL-37. Furthermore, we show that AD whole brain homogenates have significantly higher antimicrobial activity than aged matched non-AD samples and that AMP action correlates with tissue Abeta levels. Consistent with Abeta-mediated activity, the increased antimicrobial action was ablated by immunodepletion of AD brain homogenates with anti-Abeta antibodies.
Conclusions/significance: Our findings suggest Abeta is a hitherto unrecognized AMP that may normally function in the innate immune system. This finding stands in stark contrast to current models of Abeta-mediated pathology and has important implications for ongoing and future AD treatment strategies.
Conflict of interest statement
Figures

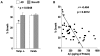
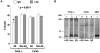
References
-
- Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer's Aβ peptide: the many roads to perdition. Neuron. 2004;43:605–608. - PubMed
-
- Koldamova RP, Lefterov IM, Lefterova MI, Lazo JS. Apolipoprotein A-I directly interacts with amyloid precursor protein and inhibits Aβ aggregation and toxicity. Biochemistry. 2001;40:3553–3560. - PubMed
-
- Maezawa I, Jin LW, Woltjer RL, Maeda N, Martin GM, et al. Apolipoprotein E isoforms and apolipoprotein AI protect from amyloid precursor protein carboxy terminal fragment-associated cytotoxicity. J Neurochem. 2004;91:1312–1321. - PubMed
-
- Zlokovic BV, Yamada S, Holtzman D, Ghiso J, Frangione B. Clearance of amyloid β-peptide from brain: transport or metabolism? Nat Med. 2000;6:718–719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

