Congenic mice confirm that collagen X is required for proper hematopoietic development
- PMID: 20209091
- PMCID: PMC2831078
- DOI: 10.1371/journal.pone.0009518
Congenic mice confirm that collagen X is required for proper hematopoietic development
Abstract
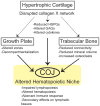
The link between endochondral skeletal development and hematopoiesis in the marrow was established in the collagen X transgenic (Tg) and null (KO) mice. Disrupted function of collagen X, a major hypertrophic cartilage matrix protein, resulted in skeletal and hematopoietic defects in endochondrally derived tissues. Manifestation of the disease phenotype was variable, ranging from perinatal lethality in a subset of mice, to altered lymphopoiesis and impaired immunity in the surviving mice. To exclude contribution of strain specific modifiers to this variable manifestation of the skeleto-hematopoietic phenotype, C57Bl/6 and DBA/2J collagen X congenic lines were established. Comparable disease manifestations confirmed that the skeleto-hematopoietic alterations are an inherent outcome of disrupted collagen X function. Further, colony forming cell assays, complete blood count analysis, serum antibody ELISA, and organ outgrowth studies established altered lymphopoiesis in all collagen X Tg and KO mice and implicated opportunistic infection as a contributor to the severe disease phenotype. These data support a model where endochondral ossification-specific collagen X contributes to the establishment of a hematopoietic niche at the chondro-osseous junction.
Conflict of interest statement
Figures




Similar articles
-
Defective endochondral ossification-derived matrix and bone cells alter the lymphopoietic niche in collagen X mouse models.Stem Cells Dev. 2013 Oct 1;22(19):2581-95. doi: 10.1089/scd.2012.0387. Epub 2013 Jun 18. Stem Cells Dev. 2013. PMID: 23656481 Free PMC article.
-
Linking hematopoiesis to endochondral skeletogenesis through analysis of mice transgenic for collagen X.Am J Pathol. 2002 Jun;160(6):2019-34. doi: 10.1016/S0002-9440(10)61152-2. Am J Pathol. 2002. PMID: 12057907 Free PMC article.
-
Altered matrix at the chondro-osseous junction leads to defects in lymphopoiesis.Ann N Y Acad Sci. 2011 Nov;1237:79-87. doi: 10.1111/j.1749-6632.2011.06227.x. Ann N Y Acad Sci. 2011. PMID: 22082369 Review.
-
A dominant interference collagen X mutation disrupts hypertrophic chondrocyte pericellular matrix and glycosaminoglycan and proteoglycan distribution in transgenic mice.Am J Pathol. 2001 Dec;159(6):2257-69. doi: 10.1016/S0002-9440(10)63076-3. Am J Pathol. 2001. PMID: 11733375 Free PMC article.
-
Altered endochondral ossification in collagen X mouse models leads to impaired immune responses.Dev Dyn. 2008 Oct;237(10):2693-704. doi: 10.1002/dvdy.21594. Dev Dyn. 2008. PMID: 18629872 Free PMC article.
Cited by
-
Detecting the Mechanism behind the Transition from Fixed Two-Dimensional Patterned Sika Deer (Cervus nippon) Dermal Papilla Cells to Three-Dimensional Pattern.Int J Mol Sci. 2021 Apr 29;22(9):4715. doi: 10.3390/ijms22094715. Int J Mol Sci. 2021. PMID: 33946876 Free PMC article.
-
Transcriptomics of Wet Skin Biopsies Predict Early Radiation-Induced Hematological Damage in a Mouse Model.Genes (Basel). 2022 Mar 18;13(3):538. doi: 10.3390/genes13030538. Genes (Basel). 2022. PMID: 35328091 Free PMC article.
-
Defective endochondral ossification-derived matrix and bone cells alter the lymphopoietic niche in collagen X mouse models.Stem Cells Dev. 2013 Oct 1;22(19):2581-95. doi: 10.1089/scd.2012.0387. Epub 2013 Jun 18. Stem Cells Dev. 2013. PMID: 23656481 Free PMC article.
-
In vitro requirement for periostin in B lymphopoiesis.Blood. 2011 Apr 7;117(14):3770-9. doi: 10.1182/blood-2010-08-301119. Epub 2011 Feb 1. Blood. 2011. PMID: 21285437 Free PMC article.
-
Aberrant expression of collagen type X in solid tumor stroma is associated with EMT, immunosuppressive and pro-metastatic pathways, bone marrow stromal cell signatures, and poor survival prognosis.BMC Cancer. 2025 Feb 12;25(1):247. doi: 10.1186/s12885-025-13641-y. BMC Cancer. 2025. PMID: 39939916 Free PMC article.
References
-
- Chan D, Jacenko O. Phenotypic and biochemical consequences of collagen X mutations in mice and humans. Matrix Biol. 1998;17:169–184. - PubMed
-
- Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res Part C: Embryo Today: Reviews. 2005;75:200–212. - PubMed
-
- Aguila HL, Rowe DW. Skeletal development, bone remodeling, and hematopoiesis. Immunol Rev. 2005;208:7–18. - PubMed
-
- Jacenko O, Ito S, Olsen BR. Skeletal and hematopoietic defects in mice transgenic for collagen X. Ann N Y Acad Sci. 1996;785:278–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

