Columnar connectivity and laminar processing in cat primary auditory cortex
- PMID: 20209092
- PMCID: PMC2831079
- DOI: 10.1371/journal.pone.0009521
Columnar connectivity and laminar processing in cat primary auditory cortex
Abstract
Background: Radial intra- and interlaminar connections form a basic microcircuit in primary auditory cortex (AI) that extracts acoustic information and distributes it to cortical and subcortical networks. Though the structure of this microcircuit is known, we do not know how the functional connectivity between layers relates to laminar processing.
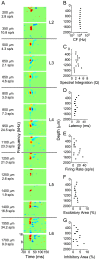
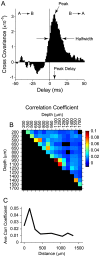
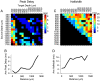
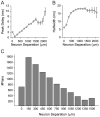
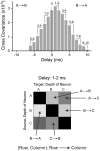
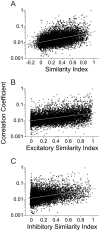
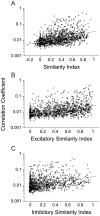
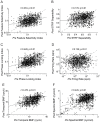
Methodology/principal findings: We studied the relationships between functional connectivity and receptive field properties in this columnar microcircuit by simultaneously recording from single neurons in cat AI in response to broadband dynamic moving ripple stimuli. We used spectrotemporal receptive fields (STRFs) to estimate the relationship between receptive field parameters and the functional connectivity between pairs of neurons. Interlaminar connectivity obtained through cross-covariance analysis reflected a consistent pattern of information flow from thalamic input layers to cortical output layers. Connection strength and STRF similarity were greatest for intralaminar neuron pairs and in supragranular layers and weaker for interlaminar projections. Interlaminar connection strength co-varied with several STRF parameters: feature selectivity, phase locking to the stimulus envelope, best temporal modulation frequency, and best spectral modulation frequency. Connectivity properties and receptive field relationships differed for vertical and horizontal connections.
Conclusions/significance: Thus, the mode of local processing in supragranular layers differs from that in infragranular layers. Therefore, specific connectivity patterns in the auditory cortex shape the flow of information and constrain how spectrotemporal processing transformations progress in the canonical columnar auditory microcircuit.
Conflict of interest statement
Figures





References
-
- Phillips DP, Irvine DR. Responses of single neurons in physiologically defined primary auditory cortex (AI) of the cat: frequency tuning and responses to intensity. J Neurophysiol. 1981;45:48–58. - PubMed
-
- Szymanski FD, Garcia-Lazaro JA, Schnupp JW. Current source density profiles of stimulus-specific adaptation in rat auditory cortex. J Neurophysiol. 2009;102:1483–1490. - PubMed
-
- Kaur S, Rose HJ, Lazar R, Liang K, Metherate R. Spectral integration in primary auditory cortex: laminar processing of afferent input, in vivo and in vitro. Neuroscience. 2005;134:1033–1045. - PubMed
-
- Wallace MN, Palmer AR. Laminar differences in the response properties of cells in the primary auditory cortex. Exp Brain Res. 2008;184:179–191. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

