A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection
- PMID: 20209097
- PMCID: PMC2831996
- DOI: 10.1371/journal.pone.0009504
A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection
Erratum in
- PLoS One. 2010;5(3). doi: 10.1371/annotation/d15b793c-85c7-4529-bc80-aabcb088a8cf
Abstract
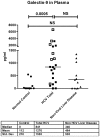
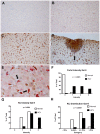
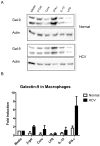
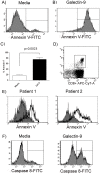
Approximately 200 million people throughout the world are infected with hepatitis C virus (HCV). One of the most striking features of HCV infection is its high propensity to establish persistence (approximately 70-80%) and progressive liver injury. Galectins are evolutionarily conserved glycan-binding proteins with diverse roles in innate and adaptive immune responses. Here, we demonstrate that galectin-9, the natural ligand for the T cell immunoglobulin domain and mucin domain protein 3 (Tim-3), circulates at very high levels in the serum and its hepatic expression (particularly on Kupffer cells) is significantly increased in patients with chronic HCV as compared to normal controls. Galectin-9 production from monocytes and macrophages is induced by IFN-gamma, which has been shown to be elevated in chronic HCV infection. In turn, galectin-9 induces pro-inflammatory cytokines in liver-derived and peripheral mononuclear cells; galectin-9 also induces anti-inflammatory cytokines from peripheral but not hepatic mononuclear cells. Galectin-9 results in expansion of CD4(+)CD25(+)FoxP3(+)CD127(low) regulatory T cells, contraction of CD4(+) effector T cells, and apoptosis of HCV-specific CTLs. In conclusion, galectin-9 production by Kupffer cells links the innate and adaptive immune response, providing a potential novel immunotherapeutic target in this common viral infection.
Conflict of interest statement
Figures
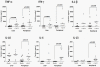


References
-
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. - PubMed
-
- Wang CC, Krantz E, Klarquist J, Krows M, McBride L, et al. Acute hepatitis C in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J Infect Dis. 2007;196:1474–1482. - PubMed
-
- Kim WR, Brown RS, Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. - PubMed
-
- Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

