Novel siRNA delivery system to target podocytes in vivo
- PMID: 20209128
- PMCID: PMC2830889
- DOI: 10.1371/journal.pone.0009463
Novel siRNA delivery system to target podocytes in vivo
Abstract
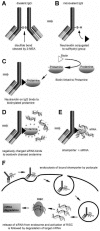
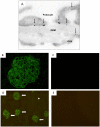
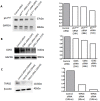
Podocytes are injured in several glomerular diseases. To alter gene expression specifically in podocytes in vivo, we took advantage of their active endocytotic machinery and developed a method for the targeted delivery of small interfering ribonucleic acids (siRNA). We generated an anti-mouse podocyte antibody that binds to rat and mouse podocytes in vivo. The polyclonal IgG antibody was cleaved into monovalent fragments, while preserving the antigen recognition sites. One Neutravidin molecule was linked to each monovalent IgG via the available sulfohydryl group. Protamine, a polycationic nuclear protein and universal adaptor for anionic siRNA, was linked to the neutravidin via biotin. The delivery system was named shamporter (sheep anti mouse podocyte transporter). Injection of shamporter coupled with either nephrin siRNA or TRPC6 siRNA via tail vein into normal rats substantially reduced the protein levels of nephrin or TRPC6 respectively, measured by western blot analysis and immunostaining. The effect was target specific because other podocyte-specific genes remained unchanged. Shamporter + nephrin siRNA induced transient proteinuria in rats. Control rats injected with shamporter coupled to control-siRNA showed no changes. These results show for the first time that siRNA can be delivered efficiently and specifically to podocytes in vivo using an antibody-delivery system.
Conflict of interest statement
Figures




Similar articles
-
ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: role of podocin and reactive oxygen species.Am J Physiol Renal Physiol. 2014 May 1;306(9):F1088-97. doi: 10.1152/ajprenal.00661.2013. Epub 2014 Feb 19. Am J Physiol Renal Physiol. 2014. PMID: 24553432 Free PMC article.
-
Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model.Biol Blood Marrow Transplant. 2013 Apr;19(4):538-46. doi: 10.1016/j.bbmt.2013.01.001. Epub 2013 Jan 4. Biol Blood Marrow Transplant. 2013. PMID: 23295166
-
Calcium entry via TRPC6 mediates albumin overload-induced endoplasmic reticulum stress and apoptosis in podocytes.Cell Calcium. 2011 Dec;50(6):523-9. doi: 10.1016/j.ceca.2011.08.008. Epub 2011 Sep 29. Cell Calcium. 2011. PMID: 21959089
-
ATRA induces podocyte differentiation and alters nephrin and podocin expression in vitro and in vivo.Kidney Int. 2005 Jul;68(1):133-44. doi: 10.1111/j.1523-1755.2005.00387.x. Kidney Int. 2005. PMID: 15954902
-
Novel role of NOD2 in mediating Ca2+ signaling: evidence from NOD2-regulated podocyte TRPC6 channels in hyperhomocysteinemia.Hypertension. 2013 Sep;62(3):506-11. doi: 10.1161/HYPERTENSIONAHA.113.01638. Epub 2013 Jul 15. Hypertension. 2013. PMID: 23856489
Cited by
-
Diverse origins of the myofibroblast—implications for kidney fibrosis.Nat Rev Nephrol. 2015 Apr;11(4):233-44. doi: 10.1038/nrneph.2014.246. Epub 2015 Jan 13. Nat Rev Nephrol. 2015. PMID: 25584804 Review.
-
Improved intratumoral delivery of PEG-coated siRNA-lipoplexes by combination with metronomic S-1 dosing in a murine solid tumor model.Drug Deliv Transl Res. 2012 Apr;2(2):77-86. doi: 10.1007/s13346-012-0059-1. Drug Deliv Transl Res. 2012. PMID: 25786716
-
Targeting Rapamycin to Podocytes Using a Vascular Cell Adhesion Molecule-1 (VCAM-1)-Harnessed SAINT-Based Lipid Carrier System.PLoS One. 2015 Sep 25;10(9):e0138870. doi: 10.1371/journal.pone.0138870. eCollection 2015. PLoS One. 2015. PMID: 26407295 Free PMC article.
-
Advances in siRNA Drug Delivery Strategies for Targeted TNBC Therapy.Bioengineering (Basel). 2024 Aug 14;11(8):830. doi: 10.3390/bioengineering11080830. Bioengineering (Basel). 2024. PMID: 39199788 Free PMC article. Review.
-
Megalin-mediated specific uptake of chitosan/siRNA nanoparticles in mouse kidney proximal tubule epithelial cells enables AQP1 gene silencing.Theranostics. 2014 Aug 13;4(10):1039-51. doi: 10.7150/thno.7866. eCollection 2014. Theranostics. 2014. PMID: 25157280 Free PMC article.
References
-
- Durvasula RV, Shankland SJ. Podocyte injury and targeting therapy: an update. Curr Opin Nephrol Hypertens. 2006;15:1–7. - PubMed
-
- NIDDK, USRDS Annual Data Report Reference. National Institute of Diabetes and Digestive and Kidney Diseases. 2006.
-
- Bronson SK, Smithies O. Altering mice by homologous recombination using embryonic stem cells. J Biol Chem. 1994;269:27155–27158. - PubMed
-
- Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol. 2004;15:1475–1487. - PubMed
-
- Durvasula RV, Shankland SJ. Models of glomerulonephritis. Methods Mol Med. 2003;86:47–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

