Synthesis and properties of a photoactivatable analogue of psychosine (beta-Galactosylsphingosine)
- PMID: 20209561
- PMCID: PMC3464015
- DOI: 10.1002/cmdc.201000018
Synthesis and properties of a photoactivatable analogue of psychosine (beta-Galactosylsphingosine)
Figures
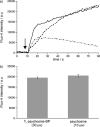


References
-
- Meyer zu Heringdorf D, Jakobs KH. Biochim. Biophys. Acta. 2007;1768:923–940. - PubMed
-
- Kanazawa T, Nakamura S, Momoi M, Yamaji T, Takematsu H, Yano H, Sabe H, Yamamoto A, Kawasaki T, Kozutsumi Y. J. Cell Biol. 2000;149:943–950. - PMC - PubMed
- Kozutsumi Y, Kanazawa T, Sun Y, Yamaji T, Yamamoto H, Takematsu H. Biochim. Biophys. Acta. 2002;1582:138–143. - PubMed
- Nilsson O, Svennerholm L. J. Neurochem. 1982;39:709–718. - PubMed
- Suzuki S. Neurochem. Res. 1998;23:251–259. - PubMed
- Sakai N. Brain Dev. 2009;31:485–487. - PubMed
- Pastores GM. Int. J. Clin. Pharmacol. Ther. 2009;47(Suppl. 1):S75–81. - PubMed
-
- Im DS, Heise CE, Nguyen T, O'Dowd BF, Lynch KR. J. Cell Biol. 2001;153:429–434. - PMC - PubMed
- Wang JQ, Kon J, Mogi C, Tobo M, Damirin A, Sato K, Komachi M, Malchinkhuu E, Murata N, Kimura T, Kuwabara A, Wakamatsu K, Koizumi H, Uede T, Tsujimoto G, Kurose H, Sato T, Harada A, Misawa N, Tomura H, Okajima F. J. Biol. Chem. 2004;279:45626–45633. - PubMed
- Tomura H, Mogi C, Sato K, Okajima F. Cell. Signalling. 2005;17:1466–1476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

