Phase 2 trial of pemetrexed disodium and carboplatin in previously untreated extensive-stage small cell lung cancer, N0423
- PMID: 20209614
- PMCID: PMC5673252
- DOI: 10.1002/cncr.24967
Phase 2 trial of pemetrexed disodium and carboplatin in previously untreated extensive-stage small cell lung cancer, N0423
Abstract
Background: In extensive-stage small cell lung cancer (SCLC), the combination of pemetrexed plus carboplatin has shown activity and appeared to be well-tolerated. We conducted a trial to confirm the efficacy and to assess the tolerability of this chemotherapy combination.
Methods: Patients with untreated extensive-stage SCLC were enrolled in this phase 2 open-labeled study. They receive pemetrexed 500 mg/m(2) and carboplatin (area under the curve of 5) every 21 days for a maximum 6 cycles. The primary endpoint for this trial was the confirmed response rate and the accrual goal was 70 patients.
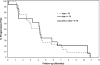
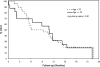
Results: Forty-six eligible patients (29 aged <70 years, 17 aged >or=70 years) were accrued to this study. The efficacy outcomes were similar between the 2 age groups. Overall, the confirmed response rate was 35% (16 of 46; 95% confidence interval [CI], 21%-50%), where all 16 were partial responses. On the basis of these results, we had strong evidence that the study would not meet the preset efficacy criteria and was, therefore, closed before full accrual. The median duration of response was 4.4 months (95% CI, 2.9-5.2). Median overall survival for patients aged <70 years and aged >or=70 years was 9.2 months (95% CI, 5.4-11.6) and 10.8 months (95% CI, 2.2-14.3), respectively. Grade 3 or higher toxicity rates were similar between the younger and older patients. Grade 3/4 and grade 4 hematological toxicities were observed in 46% and 26% of patients, respectively.
Conclusions: Although well-tolerated, the combination of pemetrexed and carboplatin is not as effective as standard therapy in patients with untreated extensive-stage SCLC.
(c) 2010 American Cancer Society.
Figures
Similar articles
-
Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-naive patients with extensive-stage small-cell lung cancer.J Clin Oncol. 2009 Oct 1;27(28):4787-92. doi: 10.1200/JCO.2009.23.1548. Epub 2009 Aug 31. J Clin Oncol. 2009. PMID: 19720897 Clinical Trial.
-
Randomized phase II trial of pemetrexed combined with either cisplatin or carboplatin in untreated extensive-stage small-cell lung cancer.J Clin Oncol. 2006 Oct 20;24(30):4840-7. doi: 10.1200/JCO.2006.07.7016. J Clin Oncol. 2006. PMID: 17050869 Clinical Trial.
-
Phase I study of carboplatin combined with pemetrexed for elderly patients with advanced non-squamous non-small cell lung cancer.Jpn J Clin Oncol. 2014 May;44(5):472-8. doi: 10.1093/jjco/hyu030. Epub 2014 Mar 30. Jpn J Clin Oncol. 2014. PMID: 24688087 Clinical Trial.
-
Pemetrexed in small-cell lung cancer: background and review of the ongoing GALES pivotal trial.Expert Rev Anticancer Ther. 2007 May;7(5):635-40. doi: 10.1586/14737140.7.5.635. Expert Rev Anticancer Ther. 2007. PMID: 17492928 Review.
-
Amrubicin: potential in combination with cisplatin or carboplatin to treat small-cell lung cancer.Drug Des Devel Ther. 2013 Aug 1;7:681-9. doi: 10.2147/DDDT.S41910. eCollection 2013. Drug Des Devel Ther. 2013. PMID: 23946645 Free PMC article. Review.
Cited by
-
First-line atezolizumab plus chemotherapy in treatment of extensive small cell lung cancer: a cost-effectiveness analysis from China.Chin Med J (Engl). 2019 Dec 5;132(23):2790-2794. doi: 10.1097/CM9.0000000000000536. Chin Med J (Engl). 2019. PMID: 31856049 Free PMC article.
-
High plasma exposure to pemetrexed leads to severe hyponatremia in patients with advanced non small cell lung cancer receiving pemetrexed-platinum doublet chemotherapy.Cancer Manag Res. 2014 Jun 4;6:261-5. doi: 10.2147/CMAR.S60177. eCollection 2014. Cancer Manag Res. 2014. PMID: 24940080 Free PMC article.
References
-
- Small Cell Lung Cancer Treatment. Available at: http://www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/HealthP.... Accessed April 9, 2009.
-
- Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3. 1973;4:31–42. - PubMed
-
- Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–1396. - PubMed
-
- Albain KS, Crowley JJ, Livingston RB. Long-term survival and toxicity in small cell lung cancer. Expanded Southwest Oncology Group experience. Chest. 1991;99:1425–1432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical