In vivo photoacoustic tomography of chemicals: high-resolution functional and molecular optical imaging at new depths
- PMID: 20210338
- PMCID: PMC2872199
- DOI: 10.1021/cr900266s
In vivo photoacoustic tomography of chemicals: high-resolution functional and molecular optical imaging at new depths
Figures


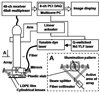



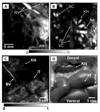


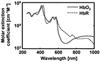






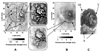



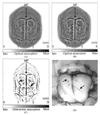

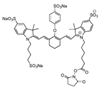


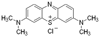
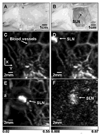

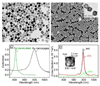

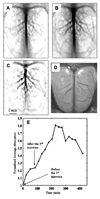
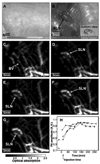


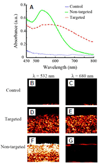

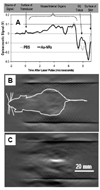




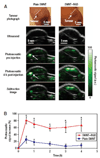
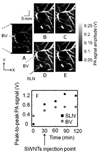
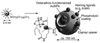




References
-
- Bell AG. Am. J. Sci. 1880;20:305.
-
- Denk W, Strickler JH, Webb WW. Science. 1990;248:73. - PubMed
-
- Singh A, Gopinathan KP. Curr. Sci. India. 1998;74:841.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

