Fluorescence lifetime in cardiovascular diagnostics
- PMID: 20210432
- PMCID: PMC2847934
- DOI: 10.1117/1.3327279
Fluorescence lifetime in cardiovascular diagnostics
Abstract
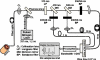
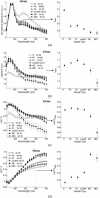
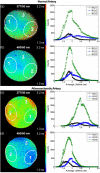
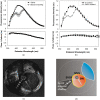
We review fluorescence lifetime techniques including time-resolved laser-induced fluorescence spectroscopy (TR-LIFS) and fluorescence lifetime imaging microscopy (FLIM) instrumentation and associated methodologies that allow for characterization and diagnosis of atherosclerotic plaques. Emphasis is placed on the translational research potential of TR-LIFS and FLIM and on determining whether intrinsic fluorescence signals can be used to provide useful contrast for the diagnosis of high-risk atherosclerotic plaque. Our results demonstrate that these techniques allow for the discrimination of important biochemical features involved in atherosclerotic plaque instability and rupture and show their potential for future intravascular applications.
Figures


Similar articles
-
Fluorescence lifetime techniques in medical applications.Ann Biomed Eng. 2012 Feb;40(2):304-31. doi: 10.1007/s10439-011-0495-y. Epub 2012 Jan 25. Ann Biomed Eng. 2012. PMID: 22273730 Free PMC article. Review.
-
Development of a dual-modal tissue diagnostic system combining time-resolved fluorescence spectroscopy and ultrasonic backscatter microscopy.Rev Sci Instrum. 2009 Jun;80(6):065104. doi: 10.1063/1.3142478. Rev Sci Instrum. 2009. PMID: 19566223 Free PMC article.
-
Laguerre-based method for analysis of time-resolved fluorescence data: application to in-vivo characterization and diagnosis of atherosclerotic lesions.J Biomed Opt. 2006 Mar-Apr;11(2):021004. doi: 10.1117/1.2186045. J Biomed Opt. 2006. PMID: 16674179 Free PMC article.
-
Diagnosis of vulnerable atherosclerotic plaques by time-resolved fluorescence spectroscopy and ultrasound imaging.Conf Proc IEEE Eng Med Biol Soc. 2006;2006:2663-6. doi: 10.1109/IEMBS.2006.259350. Conf Proc IEEE Eng Med Biol Soc. 2006. PMID: 17946129
-
Statistical filtering in fluorescence microscopy and fluorescence correlation spectroscopy.Anal Bioanal Chem. 2014 Aug;406(20):4797-813. doi: 10.1007/s00216-014-7892-7. Epub 2014 Jun 8. Anal Bioanal Chem. 2014. PMID: 24908406 Review.
Cited by
-
Multispectral scanning time-resolved fluorescence spectroscopy (TRFS) technique for intravascular diagnosis.Biomed Opt Express. 2012 Jul 1;3(7):1521-33. doi: 10.1364/BOE.3.001521. Epub 2012 Jun 6. Biomed Opt Express. 2012. PMID: 22808425 Free PMC article.
-
Fluorescence lifetime techniques in medical applications.Ann Biomed Eng. 2012 Feb;40(2):304-31. doi: 10.1007/s10439-011-0495-y. Epub 2012 Jan 25. Ann Biomed Eng. 2012. PMID: 22273730 Free PMC article. Review.
-
Application of non-negative matrix factorization to multispectral FLIM data analysis.Biomed Opt Express. 2012 Sep 1;3(9):2244-62. doi: 10.1364/BOE.3.002244. Epub 2012 Aug 24. Biomed Opt Express. 2012. PMID: 23024917 Free PMC article.
-
Multispectral fluorescence lifetime imaging system for intravascular diagnostics with ultrasound guidance: in vivo validation in swine arteries.J Biophotonics. 2014 May;7(5):281-5. doi: 10.1002/jbio.201200220. Epub 2013 Mar 13. J Biophotonics. 2014. PMID: 23495014 Free PMC article.
-
Hyperdimensional Imaging Contrast Using an Optical Fiber.Sensors (Basel). 2021 Feb 9;21(4):1201. doi: 10.3390/s21041201. Sensors (Basel). 2021. PMID: 33572130 Free PMC article.
References
-
- Naghavi M., Libby P., Falk E., Casscells S. W., Litovsky S., Rumberger J., Badimon J. J., Stefanadis C., Moreno P., Pasterkamp G., Fayad Z., Stone P. H., Waxman S., Raggi P., Madjid M., Zarrabi A., Burke A., Yuan C., Fitzgerald P. J., Siscovick D. S., de Korte C. L., Aikawa M., Juhani Airaksinen K. E., Assmann G., Becker C. R., Chesebro J. H., Farb A., Galis Z. S., Jackson C., Jang I. K., Koenig W., Lodder R. A., March K., Demirovic J., Navab M., Priori S. G., Rekhter M. D., Bahr R., Grundy S. M., Mehran R., Colombo A., Boerwinkle E., Ballantyne C., W.InsullJr., Schwartz R. S., Vogel R., Serruys P. W., Hansson G. K., Faxon D. P., Kaul S., Drexler H., Greenland P., Muller J. E., Virmani R., Ridker P. M., Zipes D. P., Shah P. K., and Willerson J. T., “From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I,” Circulation CIRCAZ 108(14), 1664–1672 (2003).10.1161/01.CIR.0000087480.94275.97 - DOI - PubMed
-
- MacNeill B. D., Lowe H. C., Takano M., Fuster V., and Jang I. K., “Intravascular modalities for detection of vulnerable plaque: current status,” Arterioscler., Thromb., Vasc. Biol. ATVBFA 23(8), 1333–1342 (2003).10.1161/01.ATV.0000080948.08888.BF - DOI - PubMed
-
- Tearney G. J., Yabushita H., Houser S. L., Aretz H. T., Jang I. K., Schlendorf K. H., Kauffman C. R., Shishkov M., Halpern E. F., and Bouma B. E., “Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography,” Circulation CIRCAZ 107(1), 113–119 (2003).10.1161/01.CIR.0000044384.41037.43 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

