Linking optics and mechanics in an in vivo model of airway fibrosis and epithelial injury
- PMID: 20210444
- PMCID: PMC2844131
- DOI: 10.1117/1.3322296
Linking optics and mechanics in an in vivo model of airway fibrosis and epithelial injury
Abstract
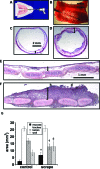
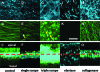
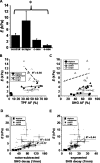
Chronic mucosal and submucosal injury can lead to persistent inflammation and tissue remodeling. We hypothesized that microstructural and mechanical properties of the airway wall could be derived from multiphoton images. New Zealand White rabbits were intubated, and the tracheal epithelium gently denuded every other day for five days (three injuries). Three days following the last injury, the tracheas were excised for multiphoton imaging, mechanical compression testing, and histological analysis. Multiphoton imaging and histology confirm epithelial denudation, mucosal ulceration, subepithelial thickening, collagen deposition, immune cell infiltration, and a disrupted elastin network. Elastase removes the elastin network and relaxes the collagen network. Purified collagenase removes epithelium with subtle subepithelial changes. Young's modulus [(E) measured in kiloPascal] was significantly elevated for the scrape injured (9.0+/-3.2) trachea, and both collagenase (2.6+/-0.4) and elastase (0.8+/-0.3) treatment significantly reduced E relative to control (4.1+/-0.7). E correlates strongly with second harmonic generation (SHG) signal depth decay for enzyme-treated and control tracheas (R(2)=0.77), but not with scrape-injured tracheas. We conclude that E of subepithelial connective tissue increases on repeated epithelial wounding, due in part to changes in elastin and collagen microstructure and concentration. SHG depth decay is sensitive to changes in extracellular matrix content and correlates with bulk Young's modulus.
Figures


Similar articles
-
Optical imaging predicts mechanical properties during decellularization of cardiac tissue.Tissue Eng Part C Methods. 2013 Oct;19(10):802-9. doi: 10.1089/ten.TEC.2012.0720. Epub 2013 May 1. Tissue Eng Part C Methods. 2013. PMID: 23469868 Free PMC article.
-
Predicting bulk mechanical properties of cellularized collagen gels using multiphoton microscopy.Acta Biomater. 2010 Dec;6(12):4657-65. doi: 10.1016/j.actbio.2010.07.004. Epub 2010 Jul 8. Acta Biomater. 2010. PMID: 20620246 Free PMC article.
-
Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton microscopy.Biophys J. 2007 Mar 15;92(6):2212-22. doi: 10.1529/biophysj.106.097998. Epub 2006 Dec 15. Biophys J. 2007. PMID: 17172303 Free PMC article.
-
Quantitative Assessment of Tendon Hierarchical Structure by Combined Second Harmonic Generation and Immunofluorescence Microscopy.Tissue Eng Part C Methods. 2020 May;26(5):253-262. doi: 10.1089/ten.TEC.2020.0032. Epub 2020 May 5. Tissue Eng Part C Methods. 2020. PMID: 32228165
-
Liver fibrosis analysis using digital pathology.Med Mol Morphol. 2024 Sep;57(3):161-166. doi: 10.1007/s00795-024-00395-y. Epub 2024 Jul 9. Med Mol Morphol. 2024. PMID: 38980407 Review.
Cited by
-
Dichotomous mechanisms of aortic stiffening in high-fat diet fed young and old B6D2F1 mice.Physiol Rep. 2014 Mar 27;2(3):e00268. doi: 10.1002/phy2.268. Print 2014. Physiol Rep. 2014. PMID: 24760522 Free PMC article.
-
Lifelong SIRT-1 overexpression attenuates large artery stiffening with advancing age.Aging (Albany NY). 2020 Jun 20;12(12):11314-11324. doi: 10.18632/aging.103322. Epub 2020 Jun 20. Aging (Albany NY). 2020. PMID: 32564006 Free PMC article.
-
Nonlinear optical microscopy and ultrasound imaging of human cervical structure.J Biomed Opt. 2013 Mar;18(3):031110. doi: 10.1117/1.JBO.18.3.031110. J Biomed Opt. 2013. PMID: 23412434 Free PMC article.
-
Mucosa-interfacing electronics.Nat Rev Mater. 2022;7(11):908-925. doi: 10.1038/s41578-022-00477-2. Epub 2022 Sep 14. Nat Rev Mater. 2022. PMID: 36124042 Free PMC article. Review.
-
Optical imaging predicts mechanical properties during decellularization of cardiac tissue.Tissue Eng Part C Methods. 2013 Oct;19(10):802-9. doi: 10.1089/ten.TEC.2012.0720. Epub 2013 May 1. Tissue Eng Part C Methods. 2013. PMID: 23469868 Free PMC article.
References
-
- Morishima Y., Nomura A., Uchida Y., Noguchi Y., Sakamoto T., Ishii Y., Goto Y., Masuyama K., Zhang M. J., Hirano K., Mochizuki M., Ohtsuka M., and Sekizawa K., “Triggering the induction of myofibroblast and fibrogenesis by airway epithelial shedding,” Am. J. Respir. Cell Mol. Biol. AJRBEL 24(1), 1–11 (2001). - PubMed
-
- Zhang S., Smartt H., Holgate S. T., and Roche W. R., “Growth factors secreted by bronchial epithelial cells control myofibroblast proliferation: an in vitro co-culture model of airway remodeling in asthma,” Lab. Invest. LAINAW 79(4), 395–405 (1999). - PubMed

