Kinetics of a single cross-bridge in familial hypertrophic cardiomyopathy heart muscle measured by reverse Kretschmann fluorescence
- PMID: 20210485
- PMCID: PMC2847936
- DOI: 10.1117/1.3324871
Kinetics of a single cross-bridge in familial hypertrophic cardiomyopathy heart muscle measured by reverse Kretschmann fluorescence
Abstract
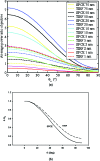
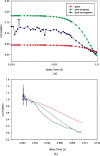
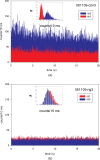
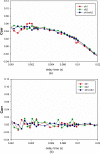
Familial hypertrophic cardiomyopathy (FHC) is a serious heart disease that often leads to a sudden cardiac death of young athletes. It is believed that the alteration of the kinetics of interaction between actin and myosin causes FHC by making the heart to pump blood inefficiently. We set out to check this hypothesis ex vivo. During contraction of heart muscle, a myosin cross-bridge imparts periodic force impulses to actin. The impulses are analyzed by fluorescence correlation spectroscopy (FCS) of fluorescently labeled actin. To minimize observation volume and background fluorescence, we carry out FCS measurements in surface plasmon coupled emission mode in a reverse Kretschmann configuration. Fluorescence is a result of near-field coupling of fluorophores excited in the vicinity of the metal-coated surface of a coverslip with the surface plasmons propagating in the metal. Surface plasmons decouple on opposite sides of the metal film and emit in a directional manner as far-field p-polarized radiation. We show that the rate of changes of orientation is significantly faster in contracting cardiac myofibrils of transgenic mice than wild type. These results are consistent with the fact that mutated heart muscle myosin translates actin faster in in vitro motility assays.
Figures

Similar articles
-
Cross-bridge kinetics in myofibrils containing familial hypertrophic cardiomyopathy R58Q mutation in the regulatory light chain of myosin.J Theor Biol. 2011 Sep 7;284(1):71-81. doi: 10.1016/j.jtbi.2011.06.014. Epub 2011 Jun 24. J Theor Biol. 2011. PMID: 21723297 Free PMC article.
-
Fluorescence lifetime of actin in the familial hypertrophic cardiomyopathy transgenic heart.Biochemistry. 2009 Feb 17;48(6):1264-71. doi: 10.1021/bi801629d. Biochemistry. 2009. PMID: 19159226 Free PMC article.
-
Single molecule detection approach to muscle study: kinetics of a single cross-bridge during contraction of muscle.Methods Mol Biol. 2012;875:311-34. doi: 10.1007/978-1-61779-806-1_17. Methods Mol Biol. 2012. PMID: 22573449
-
Cellular and molecular aspects of familial hypertrophic cardiomyopathy caused by mutations in the cardiac troponin I gene.Mol Cell Biochem. 2004 Aug;263(1-2):99-114. doi: 10.1023/B:MCBI.0000041852.42291.aa. Mol Cell Biochem. 2004. PMID: 15524171 Review.
-
Invited Review: pathophysiology of cardiac muscle contraction and relaxation as a result of alterations in thin filament regulation.J Appl Physiol (1985). 2001 Mar;90(3):1125-36. doi: 10.1152/jappl.2001.90.3.1125. J Appl Physiol (1985). 2001. PMID: 11181629 Review.
Cited by
-
Familial hypertrophic cardiomyopathy can be characterized by a specific pattern of orientation fluctuations of actin molecules.Biochemistry. 2010 Jun 29;49(25):5269-77. doi: 10.1021/bi1006749. Biochemistry. 2010. PMID: 20509708 Free PMC article.
-
Surface plasmon-coupled emission imaging for biological applications.Anal Bioanal Chem. 2020 Sep;412(24):6085-6100. doi: 10.1007/s00216-020-02635-3. Epub 2020 Apr 16. Anal Bioanal Chem. 2020. PMID: 32300846 Review.
-
Mesoscopic analysis of motion and conformation of cross-bridges.Biophys Rev. 2012 Dec;4(4):299-311. doi: 10.1007/s12551-012-0074-y. Epub 2012 Apr 17. Biophys Rev. 2012. PMID: 28510208 Free PMC article. Review.
-
Myosin regulatory light chain mutation found in hypertrophic cardiomyopathy patients increases isometric force production in transgenic mice.Biochem J. 2012 Feb 15;442(1):95-103. doi: 10.1042/BJ20111145. Biochem J. 2012. PMID: 22091967 Free PMC article.
References
-
- Maron B. J., Olivotto I., Spirito P., Casey S. A., Bellone P., and Gohman T. E., “Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population,” Circulation CIRCAZ 102, 858–864 (2000). - PubMed

