Redox control systems in the nucleus: mechanisms and functions
- PMID: 20210649
- PMCID: PMC2935340
- DOI: 10.1089/ars.2009.3021
Redox control systems in the nucleus: mechanisms and functions
Abstract
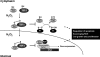
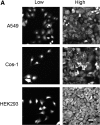
Proteins with oxidizable thiols are essential to many functions of cell nuclei, including transcription, chromatin stability, nuclear protein import and export, and DNA replication and repair. Control of the nuclear thiol-disulfide redox states involves both the elimination of oxidants to prevent oxidation and the reduction of oxidized thiols to restore function. These processes depend on the common thiol reductants, glutathione (GSH) and thioredoxin-1 (Trx1). Recent evidence shows that these systems are controlled independent of the cytoplasmic counterparts. In addition, the GSH and Trx1 couples are not in redox equilibrium, indicating that these reductants have nonredundant functions in their support of proteins involved in transcriptional regulation, nuclear protein trafficking, and DNA repair. Specific isoforms of glutathione peroxidases, glutathione S-transferases, and peroxiredoxins are enriched in nuclei, further supporting the interpretation that functions of the thiol-dependent systems in nuclei are at least quantitatively distinct, and probably also qualitatively distinct, from similar processes in the cytoplasm. Elucidation of the distinct nuclear functions and regulation of the thiol redox pathways in nuclei can be expected to improve understanding of nuclear processes and also to provide the basis for novel approaches to treat aging and disease processes associated with oxidative stress in the nuclei.
Figures



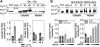



Similar articles
-
Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions.Free Radic Biol Med. 2006 Jan 1;40(1):138-45. doi: 10.1016/j.freeradbiomed.2005.09.023. Epub 2005 Oct 19. Free Radic Biol Med. 2006. PMID: 16337887
-
Compartmentation of Nrf-2 redox control: regulation of cytoplasmic activation by glutathione and DNA binding by thioredoxin-1.Toxicol Sci. 2004 Nov;82(1):308-17. doi: 10.1093/toxsci/kfh231. Epub 2004 Jul 28. Toxicol Sci. 2004. PMID: 15282410
-
Selective protection of nuclear thioredoxin-1 and glutathione redox systems against oxidation during glucose and glutamine deficiency in human colonic epithelial cells.Free Radic Biol Med. 2007 Feb 1;42(3):363-70. doi: 10.1016/j.freeradbiomed.2006.11.005. Epub 2006 Nov 10. Free Radic Biol Med. 2007. PMID: 17210449 Free PMC article.
-
Nuclear thiol redox systems in plants.Plant Sci. 2016 Feb;243:84-95. doi: 10.1016/j.plantsci.2015.12.002. Epub 2015 Dec 9. Plant Sci. 2016. PMID: 26795153 Review.
-
Glutathione--linking cell proliferation to oxidative stress.Free Radic Biol Med. 2015 Dec;89:1154-64. doi: 10.1016/j.freeradbiomed.2015.09.023. Epub 2015 Nov 3. Free Radic Biol Med. 2015. PMID: 26546102 Review.
Cited by
-
Activity-dependent Regulation of Histone Lysine Demethylase KDM1A by a Putative Thiol/Disulfide Switch.J Biol Chem. 2016 Nov 18;291(47):24756-24767. doi: 10.1074/jbc.M116.734426. Epub 2016 Sep 15. J Biol Chem. 2016. PMID: 27634040 Free PMC article.
-
Hyperoxia activates ATM independent from mitochondrial ROS and dysfunction.Redox Biol. 2015 Aug;5:176-185. doi: 10.1016/j.redox.2015.04.012. Epub 2015 May 2. Redox Biol. 2015. PMID: 25967673 Free PMC article.
-
Photooligomerization Determines Photosensitivity and Photoreactivity of Plant Cryptochromes.Mol Plant. 2020 Mar 2;13(3):398-413. doi: 10.1016/j.molp.2020.01.002. Epub 2020 Jan 14. Mol Plant. 2020. PMID: 31953223 Free PMC article.
-
Thioredoxin-1 redox signaling regulates cell survival in response to hyperoxia.Free Radic Biol Med. 2014 Oct;75:167-77. doi: 10.1016/j.freeradbiomed.2014.07.023. Epub 2014 Aug 6. Free Radic Biol Med. 2014. PMID: 25106706 Free PMC article.
-
The Sub1 nuclear protein protects DNA from oxidative damage.Mol Cell Biochem. 2016 Jan;412(1-2):165-71. doi: 10.1007/s11010-015-2621-x. Epub 2015 Dec 26. Mol Cell Biochem. 2016. PMID: 26708217 Free PMC article.
References
-
- Abate C. Patel L. Rauscher FJ., 3rd Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. - PubMed
-
- Ali-Osman F. Brunner JM. Kutluk TM. Hess K. Prognostic significance of glutathione S-transferase pi expression and subcellular localization in human gliomas. Clin Cancer Res. 1997;3:2253–2261. - PubMed
-
- Banmeyer I. Marchand C. Verhaeghe C. Vucic B. Rees JF. Knoops B. Overexpression of human peroxiredoxin 5 in subcellular compartments of Chinese hamster ovary cells: effects on cytotoxicity and DNA damage caused by peroxides. Free Radic Biol Med. 2004;36:65–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

