Performance, properties and plasticity of identified oxytocin and vasopressin neurones in vitro
- PMID: 20210845
- PMCID: PMC2910405
- DOI: 10.1111/j.1365-2826.2010.01989.x
Performance, properties and plasticity of identified oxytocin and vasopressin neurones in vitro
Abstract
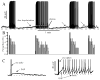
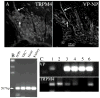
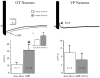
The neurohypophysial hormones oxytocin (OT) and vasopressin (VP) originate from hypothalamic neurosecretory cells in the paraventricular and supraoptic (SON) nuclei. The firing rate and pattern of action potentials arising from these neurones determine the timing and quantity of peripheral hormone release. We have used immunochemical identification of biocytin-filled SON neurones in hypothalamic slices in vitro to uncover differences between OT and VP neurones in membrane and synaptic properties, firing patterns, and plasticity during pregnancy and lactation. In this review, we summarise some recent findings from this approach: (i) VP neuronal excitability is influenced by slow (sDAP) and fast (fDAP) depolarising afterpotentials that underlie phasic bursting activity. The fDAP may relate to a transient receptor potential (TRP) channel, type melastatin (TRPM4 and/or TRPM5), both of which are immunochemically localised more to VP neurones, and especially, to their dendrites. Both TRPM4 and TRPM5 mRNAs are found in the SON, but single cell reverse transcriptase-polymerisation suggests that TRPM4 might be the more prominent channel. Phasic bursting in VP neurones is little influenced by spontaneous synaptic activity in slices, being shaped largely by intrinsic currents. (ii) The firing pattern of OT neurones ranges from irregular to continuous, with the coefficient of variation determined by randomly distributed, spontaneous GABAergic, inhibitory synaptic currents (sIPSCs). These sIPSCs are four- to five-fold more frequent in OT versus VP neurones, and much more frequent than spontaneous excitatory synaptic currents. (iii) Both cell types express Ca(2+)-dependent afterhyperpolarisations (AHPs), including an apamin-sensitive, medium duration AHP and a slower, apamin-insensitive AHP (sAHP). In OT neurones, both AHPs are enhanced during pregnancy and lactation. During pregnancy, the plasticity of the sAHP is blocked by antagonism of central OT receptors. AHP enhancement is mimicked by exposing slices from day 19 pregnant rats to OT and oestradiol, suggesting that central OT and sex steroids programme this plasticity during pregnancy by direct hypothalamic actions. In conclusion, the differences in VP and OT neuronal function are underlain by differences in both membrane and synaptic properties, and differentially modulated by reproductive state.
Figures
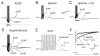
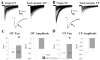
References
-
- Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. - PubMed
-
- Bicknell R. Downstream consequences of bursting activity in oxytocin neurones. In: Leng G, editor. Pulsatility in Neuroendocrine Systems. Boca Raton: CRC Press; 1988. pp. 62–74.
-
- Bicknell REJ, Leng G. Relative efficiency of neural firing patterns for vasopressin release from the rat neurohypophysis. Neuroendocrinology. 1981;33:295–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

