Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model
- PMID: 20210985
- PMCID: PMC2851702
- DOI: 10.1186/1471-2288-10-18
Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model
Abstract
Background: Antibodies directed against haemagglutinin, measured by the haemagglutination inhibition (HI) assay are essential to protective immunity against influenza infection. An HI titre of 1:40 is generally accepted to correspond to a 50% reduction in the risk of contracting influenza in a susceptible population, but limited attempts have been made to further quantify the association between HI titre and protective efficacy.
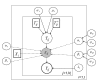
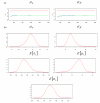
Methods: We present a model, using a meta-analytical approach, that estimates the level of clinical protection against influenza at any HI titre level. Source data were derived from a systematic literature review that identified 15 studies, representing a total of 5899 adult subjects and 1304 influenza cases with interval-censored information on HI titre. The parameters of the relationship between HI titre and clinical protection were estimated using Bayesian inference with a consideration of random effects and censorship in the available information.
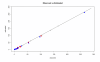
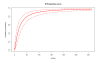
Results: A significant and positive relationship between HI titre and clinical protection against influenza was observed in all tested models. This relationship was found to be similar irrespective of the type of viral strain (A or B) and the vaccination status of the individuals.
Conclusion: Although limitations in the data used should not be overlooked, the relationship derived in this analysis provides a means to predict the efficacy of inactivated influenza vaccines when only immunogenicity data are available. This relationship can also be useful for comparing the efficacy of different influenza vaccines based on their immunological profile.
Figures
References
-
- WHO. Factsheet number 211. World Health Organization; 2008. Influenza factsheet.http://www.who.int/mediacentre/factsheets/fs211/en/index.html 14-3-2008.
-
- Palmer DF, Dowdle WR, Coleman MT, Schild GC. Advanced laboratory technicals for immunological diagnostic. US Department of Health; 1975.
-
- Committee for Proprietary Medicinal Products (CPMP) Note for Guidance on Harmonisation of Requirements for Influenza Vaccines. EMEA. 1997.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

