Alterations in the steroid hormone receptor co-chaperone FKBPL are associated with male infertility: a case-control study
- PMID: 20210997
- PMCID: PMC2844388
- DOI: 10.1186/1477-7827-8-22
Alterations in the steroid hormone receptor co-chaperone FKBPL are associated with male infertility: a case-control study
Abstract
Background: Male infertility is a common cause of reproductive failure in humans. In mice, targeted deletions of the genes coding for FKBP6 or FKBP52, members of the FK506 binding protein family, can result in male infertility. In the case of FKBP52, this reflects an important role in potentiating Androgen Receptor (AR) signalling in the prostate and accessory glands, but not the testis. In infertile men, no mutations of FKBP52 or FKBP6 have been found so far, but the gene for FKBP-like (FKBPL) maps to chromosome 6p21.3, an area linked to azoospermia in a group of Japanese patients.
Methods: To determine whether mutations in FKBPL could contribute to the azoospermic phenotype, we examined expression in mouse and human tissues by RNA array blot, RT-PCR and immunohistochemistry and sequenced the complete gene from two azoospermic patient cohorts and matching control groups. FKBPL-AR interaction was assayed using reporter constructs in vitro.
Results: FKBPL is strongly expressed in mouse testis, with expression upregulated at puberty. The protein is expressed in human testis in a pattern similar to FKBP52 and also enhanced AR transcriptional activity in reporter assays. We examined sixty patients from the Japanese patient group and found one inactivating mutation and one coding change, as well as a number of non-coding changes, all absent in fifty-six controls. A second, Irish patient cohort of thirty showed another two coding changes not present in thirty proven fertile controls.
Conclusions: Our results describe the first alterations in the gene for FKBPL in azoospermic patients and indicate a potential role in AR-mediated signalling in the testis.
Figures
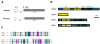

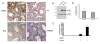
Similar articles
-
A novel FK506-like binding protein interacts with the glucocorticoid receptor and regulates steroid receptor signaling.Endocrinology. 2008 Nov;149(11):5724-34. doi: 10.1210/en.2008-0168. Epub 2008 Jul 31. Endocrinology. 2008. PMID: 18669603
-
Physiological role for the cochaperone FKBP52 in androgen receptor signaling.Mol Endocrinol. 2005 Jun;19(6):1654-66. doi: 10.1210/me.2005-0071. Epub 2005 Apr 14. Mol Endocrinol. 2005. PMID: 15831525
-
Male infertility: no evidence of involvement of androgen receptor gene among Indian men.J Androl. 2006 Jan-Feb;27(1):102-5. doi: 10.2164/jandrol.05095. J Androl. 2006. PMID: 16400085
-
The emerging role of FK506-binding proteins as cancer biomarkers: a focus on FKBPL.Biochem Soc Trans. 2011 Apr;39(2):663-8. doi: 10.1042/BST0390663. Biochem Soc Trans. 2011. PMID: 21428958 Review.
-
[Genetics and male infertility].Verh K Acad Geneeskd Belg. 2009;71(3):115-39. Verh K Acad Geneeskd Belg. 2009. PMID: 20088251 Review. Dutch.
Cited by
-
Characterisation of cardiac health in the reduced uterine perfusion pressure model and a 3D cardiac spheroid model, of preeclampsia.Biol Sex Differ. 2021 Apr 20;12(1):31. doi: 10.1186/s13293-021-00376-1. Biol Sex Differ. 2021. PMID: 33879252 Free PMC article.
-
Genome-wide association study implicates testis-sperm specific FKBP6 as a susceptibility locus for impaired acrosome reaction in stallions.PLoS Genet. 2012;8(12):e1003139. doi: 10.1371/journal.pgen.1003139. Epub 2012 Dec 20. PLoS Genet. 2012. PMID: 23284302 Free PMC article.
-
FKBPL and peptide derivatives: novel biological agents that inhibit angiogenesis by a CD44-dependent mechanism.Clin Cancer Res. 2011 Mar 1;17(5):1044-56. doi: 10.1158/1078-0432.CCR-10-2241. Epub 2011 Mar 1. Clin Cancer Res. 2011. PMID: 21364036 Free PMC article.
-
RALA-mediated delivery of FKBPL nucleic acid therapeutics.Nanomedicine (Lond). 2015 Oct;10(19):2989-3001. doi: 10.2217/nnm.15.115. Epub 2015 Sep 30. Nanomedicine (Lond). 2015. PMID: 26419658 Free PMC article.
-
Functional diversity and pharmacological profiles of the FKBPs and their complexes with small natural ligands.Cell Mol Life Sci. 2013 Sep;70(18):3243-75. doi: 10.1007/s00018-012-1206-z. Epub 2012 Dec 8. Cell Mol Life Sci. 2013. PMID: 23224428 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

