Efficacy and safety of indacaterol 150 microg once-daily in COPD: a double-blind, randomised, 12-week study
- PMID: 20211002
- PMCID: PMC2848004
- DOI: 10.1186/1471-2466-10-11
Efficacy and safety of indacaterol 150 microg once-daily in COPD: a double-blind, randomised, 12-week study
Abstract
Background: Indacaterol is a novel, once-daily (o.d.) inhaled, long-acting beta2-agonist in development for chronic obstructive pulmonary disease (COPD). This 12-week, double-blind study compared the efficacy, safety, and tolerability of indacaterol to that of placebo in patients with moderate-to-severe COPD.
Methods: Efficacy variables included 24-h trough FEV1 (mean of 23 h 10 min and 23 h 45 min post-dose) at Week 12 (primary endpoint) and after Day 1, and the percentage of COPD days with poor control (i.e., worsening symptoms). Safety was assessed by adverse events (AEs), mean serum potassium and blood glucose, QTc (Fridericia), and vital signs.
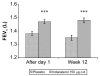
Results: Patients were randomised (n = 416, mean age 63 years) to receive either indacaterol 150 microg o.d. (n = 211) or placebo (n = 205) via a single-dose dry-powder inhaler; 87.5% completed the study. Trough FEV1 (LSM +/- SEM) at Week 12 was 1.48 +/- 0.018 L for indacaterol and 1.35 +/- 0.019 L for placebo, a clinically relevant difference of 130 +/- 24 mL (p < 0.001). Trough FEV1 after one dose was significantly higher with indacaterol than placebo (p < 0.001). Indacaterol demonstrated significantly higher peak FEV1 than placebo, both on Day 1 and at Week 12, with indacaterol-placebo differences (LSM +/- SEM) of 190 +/- 28 (p < 0.001) and 160 +/- 28 mL (p < 0.001), respectively. Standardised AUC measurements for FEV1 (between 5 min and 4 h, 5 min and 1 h, and 1 and 4 h post-dose) at Week 12 were all significantly greater with indacaterol than placebo (p < 0.001), with LSM (+/- SEM) differences of 170 +/- 24, 180 +/- 24, and 170 +/- 24 mL, respectively. Indacaterol significantly reduced the percentage of days of poor control versus placebo by 22.5% (p < 0.001) and was also associated with significantly reduced use of rescue medication (p < 0.001). The overall rates of AEs were comparable between the groups (indacaterol 49.3%, placebo 46.8%), with the most common AEs being COPD worsening (indacaterol 8.5%, placebo 12.2%) and cough (indacaterol 6.2%, placebo 7.3%). One patient died in the placebo group. Serum potassium and blood glucose levels did not differ significantly between the two groups, and no patient had QTc >500 ms.
Conclusions: Indacaterol 150 microg o.d. provided clinically significant and sustained bronchodilation, reduced rescue medication use, and had a safety and tolerability profile similar to placebo.
Trial registration: NCT00624286.
Figures

References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.com Updated 2008. [Accessed: 04 March 2010] - PubMed
-
- Dahl R, Greefhorst LA, Nowak D, Nonikov V, Byrne AM, Thomson MH, Till D, Della Cioppa G. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):778–784. - PubMed
-
- Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD) Eur Respir J. 1997;10(4):815–821. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

