Nitric oxide blocks cellular heme insertion into a broad range of heme proteins
- PMID: 20211245
- PMCID: PMC2866197
- DOI: 10.1016/j.freeradbiomed.2010.02.038
Nitric oxide blocks cellular heme insertion into a broad range of heme proteins
Abstract
Although the insertion of heme into proteins enables their function in bioenergetics, metabolism, and signaling, the mechanisms and regulation of this process are not fully understood. We developed a means to study cellular heme insertion into apo-protein targets over a 3-h period and then investigated how nitric oxide (NO) released from a chemical donor (NOC-18) might influence heme (protoporphyrin IX) insertion into seven targets that present a range of protein structures, heme ligation states, and functions (three NO synthases, two cytochrome P450's, catalase, and hemoglobin). NO blocked cellular heme insertion into all seven apo-protein targets. The inhibition occurred at relatively low (nM/min) fluxes of NO, was reversible, and did not involve changes in intracellular heme levels, activation of guanylate cyclase, or inhibition of mitochondrial ATP production. These aspects and the range of protein targets suggest that NO can act as a global inhibitor of heme insertion, possibly by inhibiting a common step in the process.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

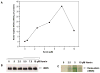
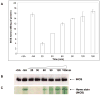
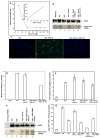





Similar articles
-
GAPDH regulates cellular heme insertion into inducible nitric oxide synthase.Proc Natl Acad Sci U S A. 2010 Oct 19;107(42):18004-9. doi: 10.1073/pnas.1008133107. Epub 2010 Oct 4. Proc Natl Acad Sci U S A. 2010. PMID: 20921417 Free PMC article.
-
Activation of purified soluble guanylate cyclase by protoporphyrin IX.Proc Natl Acad Sci U S A. 1982 May;79(9):2870-3. doi: 10.1073/pnas.79.9.2870. Proc Natl Acad Sci U S A. 1982. PMID: 6123998 Free PMC article.
-
Activation of soluble guanylate cyclase by NO-hemoproteins involves NO-heme exchange. Comparison of heme-containing and heme-deficient enzyme forms.J Biol Chem. 1986 Apr 15;261(11):4997-5002. J Biol Chem. 1986. PMID: 2870064
-
Nitric oxide and intracellular heme.Adv Pharmacol. 1995;34:277-91. doi: 10.1016/s1054-3589(08)61092-3. Adv Pharmacol. 1995. PMID: 8562440 Review.
-
Hsp90 in Human Diseases: Molecular Mechanisms to Therapeutic Approaches.Cells. 2022 Mar 12;11(6):976. doi: 10.3390/cells11060976. Cells. 2022. PMID: 35326427 Free PMC article. Review.
Cited by
-
Nitric Oxide Trickle Drives Heme into Hemoglobin and Muscle Myoglobin.Cells. 2022 Sep 12;11(18):2838. doi: 10.3390/cells11182838. Cells. 2022. PMID: 36139413 Free PMC article.
-
Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation.Physiol Rev. 2019 Jan 1;99(1):311-379. doi: 10.1152/physrev.00036.2017. Physiol Rev. 2019. PMID: 30379623 Free PMC article. Review.
-
Regeneration or Risk? A Narrative Review of BPC-157 for Musculoskeletal Healing.Curr Rev Musculoskelet Med. 2025 Aug 12. doi: 10.1007/s12178-025-09990-7. Online ahead of print. Curr Rev Musculoskelet Med. 2025. PMID: 40789979 Review. No abstract available.
-
GAPDH regulates cellular heme insertion into inducible nitric oxide synthase.Proc Natl Acad Sci U S A. 2010 Oct 19;107(42):18004-9. doi: 10.1073/pnas.1008133107. Epub 2010 Oct 4. Proc Natl Acad Sci U S A. 2010. PMID: 20921417 Free PMC article.
-
Glyceraldehyde-3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells.J Biol Chem. 2018 Sep 14;293(37):14557-14568. doi: 10.1074/jbc.RA118.004169. Epub 2018 Jul 16. J Biol Chem. 2018. PMID: 30012884 Free PMC article.
References
-
- Raven E, Ortiz de Montellano PR. Editorial: The chemistry and biochemistry of heme proteins. Nat Prod Rep. 2007;24:499.
-
- Paoli M, Marles-Wright J, Smith A. Structure-function relationships in heme-proteins. DNA Cell Biol. 2002;21:271–280. - PubMed
-
- Poulos TL. Structural biology of heme monooxygenases. Biochem Biophys Res Commun. 2005;338:337–345. - PubMed
-
- Zhu Y, Silverman RB. Revisiting heme mechanisms. A perspective on the mechanisms of nitric oxide synthase (NOS), Heme oxygenase (HO), and cytochrome P450s (CYP450s) Biochemistry. 2008;47:2231–2243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

