Isolation and characterization of novel pituitary tumor related genes: a cDNA representational difference approach
- PMID: 20211686
- PMCID: PMC2904873
- DOI: 10.1016/j.mce.2010.02.040
Isolation and characterization of novel pituitary tumor related genes: a cDNA representational difference approach
Abstract
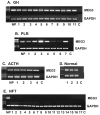
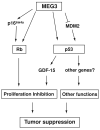
Recently, progress has been made in understanding human pituitary tumor pathogenesis by the investigation of differences in gene expression between normal pituitary tissue and pituitary tumors. A number of approaches, including differential display (DD), representational difference analysis (RDA), and microarray analysis have been used and several molecular targets potentially associated with pituitary tumor development and invasion have been identified. We have used RDA to compare gene expression patterns between normal human pituitary and clinically non-functioning pituitary adenomas, and identified genes with growth suppression function which are expressed in the normal pituitary but not in pituitary tumors. In particular, we have focused on an imprinted gene, Maternally Expressed Gene 3 (MEG3), which is specifically associated with clinically non-functioning pituitary adenomas of a gonadotroph lineage. MEG3 functions to suppress tumor cell growth, increase protein expression of the tumor suppressor p53, and selectively activate p53 target genes. Interestingly, MEG3 does not encode a protein but a non-coding RNA. Therefore, these studies have revealed novel mechanisms for the function of a non-coding RNA in pituitary physiology and tumorigenesis.
2010 Elsevier Ireland Ltd. All rights reserved.
Figures


References
-
- Biller BM, Alexander JM, Zervas NT, Hedley-Whyte ET, Arnold A, Klibanski A. Clonal origins of adrenocorticotropin-secreting pituitary tissue in Cushing's disease. Journal of Clinical Endocrinology & Metabolism. 1992;75:1303–9. - PubMed
-
- Herman V, Drazin NZ, Gonsky R, Melmed S. Molecular screening of pituitary adenomas for gene mutations and rearrangements. Journal of Clinical Endocrinology & Metabolism. 1993;77:50–5. - PubMed
-
- Levy A, Hall L, Yeudall WA, Lightman SL. p53 gene mutations in pituitary adenomas: rare events. Clinical Endocrinology. 1994;41:809–14. - PubMed
-
- Boggild MD, Jenkinson S, Pistorello M, Boscaro M, Scanarini M, McTernan P, Perrett CW, Thakker RV, Clayton RN. Molecular genetic studies of sporadic pituitary tumors. Journal of Clinical Endocrinology & Metabolism. 1994;78:387–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

