An avian model for the reversal of neurobehavioral teratogenicity with neural stem cells
- PMID: 20211723
- PMCID: PMC2875338
- DOI: 10.1016/j.ntt.2010.02.003
An avian model for the reversal of neurobehavioral teratogenicity with neural stem cells
Abstract
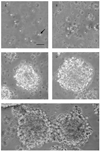
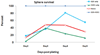
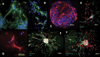
A fast and simple model which uses lower animals on the evolutionary scale is beneficial for developing procedures for the reversal of neurobehavioral teratogenicity with neural stem cells. Here, we established a procedure for the derivation of chick neural stem cells, establishing embryonic day (E) 10 as optimal for progression to neuronal phenotypes. Cells were obtained from the embryonic cerebral hemispheres and incubated for 5-7 days in enriched medium containing epidermal growth factor (EGF) and basic fibroblast growth factor (FGF2) according to a procedure originally developed for mice. A small percentage of the cells survived, proliferated and formed nestin-positive neurospheres. After removal of the growth factors to allow differentiation (5 days), 74% of the cells differentiated into all major lineages of the nervous system, including neurons (Beta III tubulin-positive, 54% of the total number of differentiated cells), astrocytes (GFAP-positive, 26%), and oligodendrocytes (O4-positive, 20%). These findings demonstrate that the cells were indeed neural stem cells. Next, the cells were transplanted in two allograft chick models; (1) direct cerebral transplantation to 24-h-old chicks, followed by post-transplantation cell tracking at 24 h, 6 days and 14 days, and (2) intravenous transplantation to chick embryos on E13, followed by cell tracking on E19. With both methods, transplanted cells were found in the brain. The chick embryo provides a convenient, precisely-timed and unlimited supply of neural progenitors for therapy by transplantation, as well as constituting a fast and simple model in which to evaluate the ability of neural stem cell transplantation to repair neural damage, steps that are critical for progress toward therapeutic applications.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Survival, differentiation, and reversal of heroin neurobehavioral teratogenicity in mice by transplanted neural stem cells derived from embryonic stem cells.J Neurosci Res. 2010 Feb 1;88(2):315-23. doi: 10.1002/jnr.22193. J Neurosci Res. 2010. PMID: 19746435
-
Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation.J Neurosci. 2003 Jan 1;23(1):240-51. doi: 10.1523/JNEUROSCI.23-01-00240.2003. J Neurosci. 2003. PMID: 12514221 Free PMC article.
-
A method for rapid derivation and propagation of neural progenitors from human embryonic stem cells.J Neurosci Methods. 2009 Nov 15;184(2):275-84. doi: 10.1016/j.jneumeth.2009.08.015. Epub 2009 Aug 26. J Neurosci Methods. 2009. PMID: 19715727
-
Establishment and properties of a growth factor-dependent, perpetual neural stem cell line from the human CNS.Exp Neurol. 2000 Jan;161(1):67-84. doi: 10.1006/exnr.1999.7237. Exp Neurol. 2000. PMID: 10683274
-
Human embryonic stem cells and lung regeneration.Br J Pharmacol. 2008 Oct;155(3):316-25. doi: 10.1038/bjp.2008.333. Epub 2008 Aug 25. Br J Pharmacol. 2008. PMID: 18724383 Free PMC article. Review.
Cited by
-
Diverse neurotoxicants target the differentiation of embryonic neural stem cells into neuronal and glial phenotypes.Toxicology. 2016 Nov 30;372:42-51. doi: 10.1016/j.tox.2016.10.015. Epub 2016 Nov 2. Toxicology. 2016. PMID: 27816694 Free PMC article.
-
Establishment of a survival and toxic cellular model for Parkinson's disease from chicken mesencephalon.Neurotox Res. 2013 Aug;24(2):119-29. doi: 10.1007/s12640-012-9367-y. Epub 2012 Dec 13. Neurotox Res. 2013. PMID: 23238634 Free PMC article.
-
Differentiation of human breast-milk stem cells to neural stem cells and neurons.Neurol Res Int. 2014;2014:807896. doi: 10.1155/2014/807896. Epub 2014 Nov 25. Neurol Res Int. 2014. PMID: 25506428 Free PMC article.
References
-
- Ambalavanar R, McCabe BJ, Potter KN, Horn G. Learning-related fos-like immunoreactivity in the chick brain: time-course and co-localization with GABA and parvalbumin. Neuroscience. 1999;93:1515–1524. - PubMed
-
- Barnabe-Heider F, Frisen J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3:16–24. - PubMed
-
- Barron S, Kelly SJ, Riley EP. Neonatal alcohol exposure alters suckling behavior in neonatal rat pups. Pharmacol Biochem Behav. 1991;39:423–427. - PubMed
-
- Ben-Hur T. Immunomodulation by neural stem cells. J Neurol Sci. 2008;265:102–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

