Ameloblast differentiation in the human developing tooth: effects of extracellular matrices
- PMID: 20211728
- PMCID: PMC3296366
- DOI: 10.1016/j.matbio.2010.03.001
Ameloblast differentiation in the human developing tooth: effects of extracellular matrices
Abstract
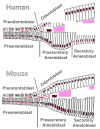
Tooth enamel is formed by epithelially-derived cells called ameloblasts, while the pulp dentin complex is formed by the dental mesenchyme. These tissues differentiate with reciprocal signaling interactions to form a mature tooth. In this study we have characterized ameloblast differentiation in human developing incisors, and have further investigated the role of extracellular matrix proteins on ameloblast differentiation. Histological and immunohistochemical analyses showed that in the human tooth, the basement membrane separating the early developing dental epithelium and mesenchyme was lost shortly before dentin deposition was initiated, prior to enamel matrix secretion. Presecretary ameloblasts elongated as they came into contact with the dentin matrix, and then shortened to become secretory ameloblasts. In situ hybridization showed that the presecretory stage of odontoblasts started to express type I collagen mRNA, and also briefly expressed amelogenin mRNA. This was followed by upregulation of amelogenin mRNA expression in secretory ameloblasts. In vitro, amelogenin expression was upregulated in ameloblast lineage cells cultured in Matrigel, and was further up-regulated when these cells/Matrigel were co-cultured with dental pulp cells. Co-culture also up-regulated type I collagen expression by the dental pulp cells. Type I collagen coated culture dishes promoted a more elongated ameloblast lineage cell morphology and enhanced cell adhesion via integrin alpha2beta1. Taken together, these results suggest that the basement membrane proteins and signals from underlying mesenchymal cells coordinate to initiate differentiation of preameloblasts and regulate type I collagen expression by odontoblasts. Type I collagen in the dentin matrix then anchors the presecretary ameloblasts as they further differentiate to secretory cells. These studies show the critical roles of the extracellular matrix proteins in ameloblast differentiation.
Copyright (c) 2010 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Two related low molecular mass polypeptide isoforms of amelogenin have distinct activities in mouse tooth germ differentiation in vitro.J Bone Miner Res. 2005 Feb;20(2):341-9. doi: 10.1359/JBMR.041107. Epub 2004 Nov 16. J Bone Miner Res. 2005. PMID: 15647828
-
Spatial distribution of enamel proteins and fibronectin at early stages of rat incisor tooth formation.Arch Oral Biol. 1995 Nov;40(11):1029-38. doi: 10.1016/0003-9969(95)00073-x. Arch Oral Biol. 1995. PMID: 8670021
-
Leucine rich amelogenin peptide alters ameloblast differentiation in vivo.Matrix Biol. 2013 Oct-Nov;32(7-8):432-42. doi: 10.1016/j.matbio.2013.05.004. Epub 2013 Jun 4. Matrix Biol. 2013. PMID: 23747796 Free PMC article.
-
Essential roles of ameloblastin in maintaining ameloblast differentiation and enamel formation.Cells Tissues Organs. 2005;181(3-4):189-95. doi: 10.1159/000091380. Cells Tissues Organs. 2005. PMID: 16612084 Review.
-
Molecular genetics of ameloblast cell lineage.J Exp Zool B Mol Dev Evol. 2009 Jul 15;312B(5):437-44. doi: 10.1002/jez.b.21261. J Exp Zool B Mol Dev Evol. 2009. PMID: 19090561 Free PMC article. Review.
Cited by
-
Three-Dimensional Culture of Ameloblast-Originated HAT-7 Cells for Functional Modeling of Defective Tooth Enamel Formation.Front Pharmacol. 2021 Jun 2;12:682654. doi: 10.3389/fphar.2021.682654. eCollection 2021. Front Pharmacol. 2021. PMID: 34149428 Free PMC article.
-
Adenovirus gene transfer to amelogenesis imperfecta ameloblast-like cells.PLoS One. 2011;6(10):e24281. doi: 10.1371/journal.pone.0024281. Epub 2011 Oct 7. PLoS One. 2011. PMID: 22003382 Free PMC article.
-
BMP7 and EREG Contribute to the Inductive Potential of Dental Mesenchyme.Sci Rep. 2015 May 8;5:9903. doi: 10.1038/srep09903. Sci Rep. 2015. PMID: 25952286 Free PMC article.
-
The molecular basis of hereditary enamel defects in humans.J Dent Res. 2015 Jan;94(1):52-61. doi: 10.1177/0022034514556708. Epub 2014 Nov 11. J Dent Res. 2015. PMID: 25389004 Free PMC article. Review.
-
The role of the TGF-β1 signaling pathway in the process of amelogenesis.Front Physiol. 2025 Apr 9;16:1586769. doi: 10.3389/fphys.2025.1586769. eCollection 2025. Front Physiol. 2025. PMID: 40271211 Free PMC article. Review.
References
-
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. - PubMed
-
- Albrecht BE, Breitenbach U, Stühmer T, Harvey RJ, Darlison MG. In situ hybridization and reverse transcription--polymerase chain reaction studies on the expression of the GABA(C) receptor rho1- and rho2-subunit genes in avian and rat brain. Eur. J. Neurosci. 1997;9:2414–2422. - PubMed
-
- Chen J, Zhang Y, Mendoza J, DenBesten P. Calcium-mediated differentiation of ameloblast lineage cells in vitro. J. Exp. Zoolog. B. Mol. Dev. Evol. 2009;312:458–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

