A second class of nuclear receptors for oxysterols: Regulation of RORalpha and RORgamma activity by 24S-hydroxycholesterol (cerebrosterol)
- PMID: 20211758
- PMCID: PMC2886165
- DOI: 10.1016/j.bbalip.2010.02.012
A second class of nuclear receptors for oxysterols: Regulation of RORalpha and RORgamma activity by 24S-hydroxycholesterol (cerebrosterol)
Abstract
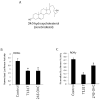
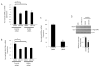
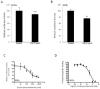
The retinoic acid receptor-related orphan receptors alpha and gamma (RORalpha [NR1F1] and RORgamma [NR1F3]) are members of the nuclear hormone receptor superfamily. These 2 receptors regulate many physiological processes including development, metabolism and immunity. We recently found that certain oxysterols, namely the 7-substituted oxysterols, bound to the ligand binding domains (LBDs) of RORalpha and RORgamma with high affinity, altered the LBD conformation and reduced coactivator binding resulting in suppression of the constitutive transcriptional activity of these two receptors. Here, we show that another oxysterol, 24S-hydroxycholesterol (24S-OHC), is also a high affinity ligand for RORalpha and RORgamma (K(i) approximately 25 nM). 24S-OHC is also known as cerebrosterol due to its high level in the brain where it plays an essential role as an intermediate in cholesterol elimination from the CNS. 24S-OHC functions as a RORalpha/gamma inverse agonist suppressing the constitutive transcriptional activity of these receptors in cotransfection assays. Additionally, 24S-OHC suppressed the expression of several RORalpha target genes including BMAL1 and REV-ERBalpha in a ROR-dependent manner. We also demonstrate that 24S-OHC decreases the ability of RORalpha to recruit the coactivator SRC-2 when bound to the BMAL1 promoter. We also noted that 24(S), 25-epoxycholesterol selectively suppressed the activity of RORgamma. These data indicate that RORalpha and RORgamma may serve as sensors of oxsterols. Thus, RORalpha and RORgamma display an overlapping ligand preference with another class of oxysterol nuclear receptors, the liver X receptors (LXRalpha [NR1H3] and LXRbeta [NR1H2]).
Copyright 2010 Elsevier B.V. All rights reserved.
Figures



References
-
- Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Current Opinion in Lipidology. 2001;12:105–112. - PubMed
-
- Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM. Activation of the Nuclear Receptor LXR by Oxysterols Defines a New Hormone Response Pathway. J Biol Chem. 1997;272:3137–3140. - PubMed
-
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

