Worldwide experience with the use of doripenem against extended-spectrum-beta-lactamase-producing and ciprofloxacin-resistant Enterobacteriaceae: analysis of six phase 3 clinical studies
- PMID: 20211892
- PMCID: PMC2863686
- DOI: 10.1128/AAC.01450-09
Worldwide experience with the use of doripenem against extended-spectrum-beta-lactamase-producing and ciprofloxacin-resistant Enterobacteriaceae: analysis of six phase 3 clinical studies
Abstract
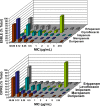
The worldwide increase in fluoroquinolone-resistant and extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae pathogens has led to doripenem and other carbapenems assuming a greater role in the treatment of serious infections. We analyzed data from 6 phase 3 multinational doripenem clinical trials on ciprofloxacin-resistant Enterobacteriaceae isolates consisting of all genera (CIPRE) and ESBL-producing Enterobacteriaceae isolates consisting of Escherichia coli, Klebsiella spp., and Proteus spp. with ceftazidime MICs of >or=2 microg/ml (ESBLE) for prevalence by geographic region and disease type, in vitro activities of doripenem and comparator agents, and clinical or microbiologic outcomes in doripenem- and comparator-treated patients across disease types (complicated intra-abdominal infection [cIAI], complicated urinary tract infection [cUTI], and nosocomial pneumonia [NP]). Of 1,830 baseline Enterobacteriaceae isolates, 88 (4.8%) were ESBLE and 238 (13.0%) were CIPRE. The incidence of ESBLE was greatest in Europe (7.8%); that of CIPRE was higher in South America (15.9%) and Europe (14.4%). ESBLE incidence was highest in NP (12.9%) cases; that of CIPRE was higher in cUTI (18.3%) and NP (14.9%) cases. Against ESBLE and CIPRE, carbapenems appeared more active than other antibiotic classes. Among carbapenems, doripenem and meropenem were most potent. Doripenem had low MIC(90)s for CIPRE (0.5 microg/ml) and ESBLE (0.25 microg/ml). Doripenem and comparators were highly clinically effective in infections caused by Enterobacteriaceae, irrespective of their ESBL statuses. The overall cure rates were the same for doripenem (82%; 564/685) and the comparators (82%; 535/652) and similar for ESBLE (73% [16/22] versus 72% [21/29]) and CIPRE (68% [47/69] versus 52% [33/64]). These findings indicate that doripenem is an important therapeutic option for treating serious infections caused by ESBLE and CIPRE.
Trial registration: ClinicalTrials.gov NCT00210938 NCT00210990 NCT00211003 NCT00211016 NCT00229021 NCT00229060.
Figures
References
-
- Chastre, J., R. Wunderink, P. Prokocimer, M. Lee, K. Kaniga, and I. Friedland. 2008. Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator-associated pneumonia: a multicenter, randomized study. Crit. Care Med. 36:1089-1096. - PubMed
-
- Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement, 19087-1898. Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Kattan, J. N., M. V. Villegas, and J. P. Quinn. 2008. New developments in carbapenems. Clin. Microbiol. Infect. 14:1102-1111. - PubMed
-
- Keam, S. J. 2008. Doripenem: a review of its use in the treatment of bacterial infections. Drugs 68:2021-2057. - PubMed
-
- Lucasti, C., A. Jasovich, O. Umeh, J. Jiang, K. Kaniga, and I. Friedland. 2008. Efficacy and tolerability of IV doripenem versus meropenem in adults with complicated intra-abdominal infection: a phase III, prospective, multicenter, randomized, double-blind, noninferiority study. Clin. Ther. 30:868-883. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous