Multi-system disorders of glycosphingolipid and ganglioside metabolism
- PMID: 20211931
- PMCID: PMC2882741
- DOI: 10.1194/jlr.R003996
Multi-system disorders of glycosphingolipid and ganglioside metabolism
Abstract
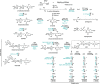
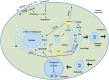
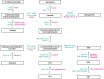
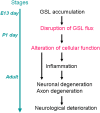
Glycosphingolipids (GSLs) and gangliosides are a group of bioactive glycolipids that include cerebrosides, globosides, and gangliosides. These lipids play major roles in signal transduction, cell adhesion, modulating growth factor/hormone receptor, antigen recognition, and protein trafficking. Specific genetic defects in lysosomal hydrolases disrupt normal GSL and ganglioside metabolism leading to their excess accumulation in cellular compartments, particularly in the lysosome, i.e., lysosomal storage diseases (LSDs). The storage diseases of GSLs and gangliosides affect all organ systems, but the central nervous system (CNS) is primarily involved in many. Current treatments can attenuate the visceral disease, but the management of CNS involvement remains an unmet medical need. Early interventions that alter the CNS disease have shown promise in delaying neurologic involvement in several CNS LSDs. Consequently, effective treatment for such devastating inherited diseases requires an understanding of the early developmental and pathological mechanisms of GSL and ganglioside flux (synthesis and degradation) that underlie the CNS diseases. These are the focus of this review.
Figures

References
-
- Holthuis J. C., Pomorski T., Raggers R. J., Sprong H., Van Meer G. 2001. The organizing potential of sphingolipids in intracellular membrane transport. Physiol. Rev. 81: 1689–1723. - PubMed
-
- Kolter T., Sandhoff K. 2005. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu. Rev. Cell Dev. Biol. 21: 81–103. - PubMed
-
- Mandon E. C., Ehses I., Rother J., van Echten G., Sandhoff K. 1992. Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase, and sphinganine N-acyltransferase in mouse liver. J. Biol. Chem. 267: 11144–11148. - PubMed
-
- Michel C., van Echten-Deckert G., Rother J., Sandhoff K., Wang E., Merrill A. H., Jr 1997. Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide. J. Biol. Chem. 272: 22432–22437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

