Outcrossing and the maintenance of males within C. elegans populations
- PMID: 20212008
- PMCID: PMC2859890
- DOI: 10.1093/jhered/esq003
Outcrossing and the maintenance of males within C. elegans populations
Abstract
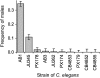
Caenorhabditis elegans is an androdioecious nematode with both hermaphrodites and males. Although males can potentially play an important role in avoiding inbreeding and facilitating adaptation, their existence is evolutionarily problematic because they do not directly generate offspring in the way that hermaphrodites do. This review explores how genetic, population genomic, and experimental evolution approaches are being used to address the role of males and outcrossing within C. elegans. Although theory suggests that inbreeding depression and male mating ability should be the primary determinants of male frequency, this has yet to be convincingly confirmed experimentally. Genomic analysis of natural populations finds that outcrossing occurs at low, but not negligible levels, and that observed patterns of linkage disequilibrium consistent with strong selfing may instead be generated by natural selection against outcrossed progeny. Recent experimental evolution studies suggest that males can be maintained at fairly high levels if populations are initiated with sufficient genetic variation and/or subjected to strong natural selection via a change in the environment. For example, as reported here, populations adapting to novel laboratory rearing and temperature regimes maintain males at frequencies from 5% to 40%. Laboratory and field results still await full reconciliation, which may be facilitated by identifying the loci underlying among-strain differences in mating system dynamics.
Figures
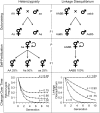
 , where s is the rate of self-fertilization and H0 is the initial heterozygosity before selfing (scaled here to its maximum value of 0.5; Crow and Kimura 1970). Linkage disequilibria at generation t are given by , where D0 is the amount of linkage disequilibrium at generation zero (scaled to 1.0 for convenience) and is the effective amount of recombination scaled by the rate of selfing within the population: (Nordborg 1997; Barrière and Félix 2005). The actual recombination rate, r, was set to 0.5 for this example.
, where s is the rate of self-fertilization and H0 is the initial heterozygosity before selfing (scaled here to its maximum value of 0.5; Crow and Kimura 1970). Linkage disequilibria at generation t are given by , where D0 is the amount of linkage disequilibrium at generation zero (scaled to 1.0 for convenience) and is the effective amount of recombination scaled by the rate of selfing within the population: (Nordborg 1997; Barrière and Félix 2005). The actual recombination rate, r, was set to 0.5 for this example.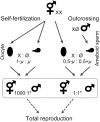


References
-
- Anderson JL, Albergotti L, Proulx S, Peden C, Huey RB, Phillips PC. Thermal preference of Caenorhabditis elegans: a null model and empirical tests. J Exp Biol. 2007;210:3107–3116. - PubMed
-
- Baldi C, Cho S, Ellis RE. Mutations in two independent pathways are sufficient to create hermaphroditic nematodes. Science. 2009;326:1002–1005. - PubMed
-
- Barker DM. Copulatory plugs and paternity assurance in the nematode Caenorhabditis elegans. Anim Behav. 1994;48:147–156.
-
- Barr MM, Garcia LR. 2006. Male mating behavior [Internet] In: The C. elegans Research Community, editor. WormBook. doi: 10.1895/wormbook.1.78.1. [cited 2006 Jun 19]. Available from: http://www.wormbook.org. - PMC - PubMed

