Mammalian Ste20-like kinase (Mst2) indirectly supports Raf-1/ERK pathway activity via maintenance of protein phosphatase-2A catalytic subunit levels and consequent suppression of inhibitory Raf-1 phosphorylation
- PMID: 20212043
- PMCID: PMC2865317
- DOI: 10.1074/jbc.M109.078915
Mammalian Ste20-like kinase (Mst2) indirectly supports Raf-1/ERK pathway activity via maintenance of protein phosphatase-2A catalytic subunit levels and consequent suppression of inhibitory Raf-1 phosphorylation
Abstract
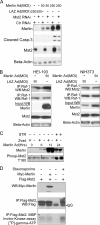
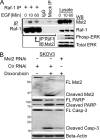
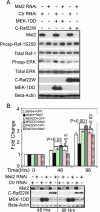
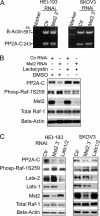
Many tumor suppressor proteins act to blunt the effects of mitogenic signaling pathways. Loss of function mutations in the merlin tumor suppressor underlie neurofibromatosis type 2 (NF2), a familial autosomal dominant cancer syndrome. Studies of Drosophila suggest that Hippo (hpo) is required for inhibition of cell proliferation mediated by dMer, the orthologue of human merlin. Mammalian sterile 20-like kinase-2 (Mst2) is a mammalian Hpo orthologue, and numerous studies implicate Mst2 as a tumor suppressor. Mst2 is negatively regulated by the proto-oncoprotein Raf-1 in a manner independent of the kinase activity of Raf-1. We sought to determine whether, in mammalian cells, merlin could positively regulate Mst2. We also sought to determine whether Mst2, in addition to being negatively regulated by Raf-1, might itself reciprocally regulate Raf-1. In contrast to findings from Drosophila, we find no evidence that mammalian merlin positively regulates mammalian Mst2. Instead, surprisingly, RNA interference silencing of Mst2 leads to elevated inhibitory phosphorylation of Raf-1 at Ser-259 and impaired Raf-1 kinase activity. Consequent to this, ERK pathway activation and cell proliferation are attenuated. Phosphatase-2A (PP2A) dephosphorylates Raf-1 Ser-259 in response to mitogens. Interestingly RNA interference silencing of Mst2 triggers a striking proteasome-dependent decrease in the levels of the catalytic subunit of PP2A (PP2A-C). A similar effect is achieved upon silencing of large tumor suppressor (LATS)-1 and LATS2, direct substrates of Mst2. Our studies reveal a more complex role for Mst2 than previously thought. The Mst2 --> LATS1/2 pathway, by maintaining PP2A-C levels, may, in some situations, positively affect mitogenic signaling.
Figures




Similar articles
-
Taming the Hippo: Raf-1 controls apoptosis by suppressing MST2/Hippo.Cell Cycle. 2005 Mar;4(3):365-7. doi: 10.4161/cc.4.3.1531. Epub 2005 Mar 8. Cell Cycle. 2005. PMID: 15701972 Review.
-
Proapoptotic kinase MST2 coordinates signaling crosstalk between RASSF1A, Raf-1, and Akt.Cancer Res. 2010 Feb 1;70(3):1195-203. doi: 10.1158/0008-5472.CAN-09-3147. Epub 2010 Jan 19. Cancer Res. 2010. PMID: 20086174 Free PMC article.
-
Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1.Science. 2004 Dec 24;306(5705):2267-70. doi: 10.1126/science.1103233. Science. 2004. PMID: 15618521
-
C. elegans SUR-6/PR55 cooperates with LET-92/protein phosphatase 2A and promotes Raf activity independently of inhibitory Akt phosphorylation sites.Development. 2004 Feb;131(4):755-65. doi: 10.1242/dev.00987. Epub 2004 Jan 14. Development. 2004. PMID: 14724126
-
Mammalian sterile 20-like kinases in tumor suppression: an emerging pathway.Cancer Res. 2005 Jul 1;65(13):5485-7. doi: 10.1158/0008-5472.CAN-05-1453. Cancer Res. 2005. PMID: 15994916 Review.
Cited by
-
Mammalian MST2 kinase and human Salvador activate and reduce estrogen receptor alpha in the absence of ligand.J Mol Med (Berl). 2011 Feb;89(2):181-91. doi: 10.1007/s00109-010-0698-y. Epub 2010 Nov 23. J Mol Med (Berl). 2011. PMID: 21104395
-
MST1/MST2 Protein Kinases: Regulation and Physiologic Roles.Biochemistry. 2016 Oct 4;55(39):5507-5519. doi: 10.1021/acs.biochem.6b00763. Epub 2016 Sep 26. Biochemistry. 2016. PMID: 27618557 Free PMC article. Review.
-
Aberrant large tumor suppressor 2 (LATS2) gene expression correlates with EGFR mutation and survival in lung adenocarcinomas.Lung Cancer. 2014 Aug;85(2):282-92. doi: 10.1016/j.lungcan.2014.05.025. Epub 2014 Jun 16. Lung Cancer. 2014. PMID: 24976335 Free PMC article.
-
Signaling pathways and clinical application of RASSF1A and SHOX2 in lung cancer.J Cancer Res Clin Oncol. 2020 Jun;146(6):1379-1393. doi: 10.1007/s00432-020-03188-9. Epub 2020 Apr 7. J Cancer Res Clin Oncol. 2020. PMID: 32266538 Free PMC article. Review.
-
Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies.Signal Transduct Target Ther. 2023 Dec 18;8(1):455. doi: 10.1038/s41392-023-01705-z. Signal Transduct Target Ther. 2023. PMID: 38105263 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

