The antibacterial cell division inhibitor PC190723 is an FtsZ polymer-stabilizing agent that induces filament assembly and condensation
- PMID: 20212044
- PMCID: PMC2863232
- DOI: 10.1074/jbc.M109.094722
The antibacterial cell division inhibitor PC190723 is an FtsZ polymer-stabilizing agent that induces filament assembly and condensation
Abstract
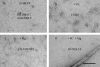
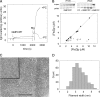
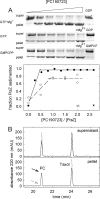
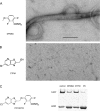
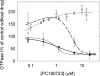
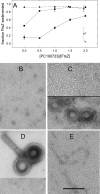
Cell division protein FtsZ can form single-stranded filaments with a cooperative behavior by self-switching assembly. Subsequent condensation and bending of FtsZ filaments are important for the formation and constriction of the cytokinetic ring. PC190723 is an effective bactericidal cell division inhibitor that targets FtsZ in the pathogen Staphylococcus aureus and Bacillus subtilis and does not affect Escherichia coli cells, which apparently binds to a zone equivalent to the binding site of the antitumor drug taxol in tubulin (Haydon, D. J., Stokes, N. R., Ure, R., Galbraith, G., Bennett, J. M., Brown, D. R., Baker, P. J., Barynin, V. V., Rice, D. W., Sedelnikova, S. E., Heal, J. R., Sheridan, J. M., Aiwale, S. T., Chauhan, P. K., Srivastava, A., Taneja, A., Collins, I., Errington, J., and Czaplewski, L. G. (2008) Science 312, 1673-1675). We have found that the benzamide derivative PC190723 is an FtsZ polymer-stabilizing agent. PC190723 induced nucleated assembly of Bs-FtsZ into single-stranded coiled protofilaments and polymorphic condensates, including bundles, coils, and toroids, whose formation could be modulated with different solution conditions. Under conditions for reversible assembly of Bs-FtsZ, PC190723 binding reduced the GTPase activity and induced the formation of straight bundles and ribbons, which was also observed with Sa-FtsZ but not with nonsusceptible Ec-FtsZ. The fragment 2,6-difluoro-3-methoxybenzamide also induced Bs-FtsZ bundling. We propose that polymer stabilization by PC190723 suppresses in vivo FtsZ polymer dynamics and bacterial division. The biochemical action of PC190723 on FtsZ parallels that of the microtubule-stabilizing agent taxol on the eukaryotic structural homologue tubulin. Both taxol and PC190723 stabilize polymers against disassembly by preferential binding to each assembled protein. It is yet to be investigated whether both ligands target structurally related assembly switches.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

