Direct positive regulation of PTEN by the p85 subunit of phosphatidylinositol 3-kinase
- PMID: 20212113
- PMCID: PMC2851819
- DOI: 10.1073/pnas.0908899107
Direct positive regulation of PTEN by the p85 subunit of phosphatidylinositol 3-kinase
Abstract
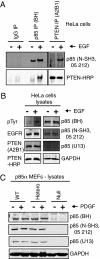
The phosphatidylinositol 3-kinase (PI3K) signaling pathway is deregulated in many human diseases including cancer, diabetes, obesity, and autoimmunity. PI3K consists of a p110 catalytic protein and a p85alpha regulatory protein, required for the stabilization and localization of p110-PI3K activity. The p110-PI3K enzyme generates the key signaling lipid phosphatidylinositol 3,4,5-trisphosphate, which is dephosphorylated by the PI3-phosphatase PTEN. Here we show another function for the p85alpha regulatory protein: it binds directly to and enhances PTEN lipid phosphatase activity. We demonstrate that ectopically expressed FLAG-tagged p85 coimmunoprecipitates endogenous PTEN in an epidermal growth factor dependent manner. We also show epidermal growth factor dependent coimmunoprecipitation of endogenous p85 and PTEN proteins in HeLa cells. Thus p85 regulates both p110-PI3K and PTEN-phosphatase enzymes through direct interaction. This finding underscores the need for caution in analyzing PI3K activity because anti-p85 immunoprecipitations may contain both p85:p110-PI3K and p85:PTEN-phosphatase enzymes and thus measure net PI3K activity. We identify the N-terminal SH3-BH region of p85alpha, absent in the smaller p55alpha and p50alpha isoforms, as the region that mediates PTEN binding and regulation. Cellular expression of p85DeltaSH3-BH results in substantially increased magnitude and duration of pAkt levels in response to growth factor stimulation. The ability of p85 to bind and directly regulate both p110-PI3K and PTEN-PI3-phosphatase allows us to explain the paradoxical insulin signaling phenotypes observed in mice with reduced PI3K or PTEN proteins. This discovery will impact ongoing studies using therapeutics targeting the PI3K/PTEN/Akt pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

