Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control
- PMID: 20212121
- PMCID: PMC2851911
- DOI: 10.1073/pnas.0911617107
Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control
Abstract
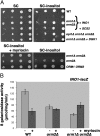
Yeast members of the ORMDL family of endoplasmic reticulum (ER) membrane proteins play a central role in lipid homeostasis and protein quality control. In the absence of yeast Orm1 and Orm2, accumulation of long chain base, a sphingolipid precursor, suggests dysregulation of sphingolipid synthesis. Physical interaction between Orm1 and Orm2 and serine palmitoyltransferase, responsible for the first committed step in sphingolipid synthesis, further supports a role for the Orm proteins in regulating sphingolipid synthesis. Phospholipid homeostasis is also affected in orm1Delta orm2Delta cells: the cells are inositol auxotrophs with impaired transcriptional regulation of genes encoding phospholipid biosynthesis enzymes. Strikingly, impaired growth of orm1Delta orm2Delta cells is associated with constitutive unfolded protein response, sensitivity to stress, and slow ER-to-Golgi transport. Inhibition of sphingolipid synthesis suppresses orm1Delta orm2Delta phenotypes, including ER stress, suggesting that disrupted sphingolipid homeostasis accounts for pleiotropic phenotypes. Thus, the yeast Orm proteins control membrane biogenesis by coordinating lipid homeostasis with protein quality control.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
Keeping sphingolipid levels nORMal.Proc Natl Acad Sci U S A. 2010 Mar 30;107(13):5701-2. doi: 10.1073/pnas.1001503107. Epub 2010 Mar 16. Proc Natl Acad Sci U S A. 2010. PMID: 20304791 Free PMC article. No abstract available.
References
-
- Hebert DN, Molinari M. In and out of the ER: Protein folding, quality control, degradation, and related human diseases. Physiol Rev. 2007;87:1377–1408. - PubMed
-
- Travers KJ, et al. Functional and genomic analysis reveal essential coordination between the unfolded protein response and endoplasmic reticulum-associated degradation. Cell. 2000;101:249–258. - PubMed
-
- Funato K, Vallée B, Riezman H. Biosynthesis and trafficking of sphingolipids in the yeast Saccharomyces cerevisiae. Biochemistry. 2002;41:15105–15114. - PubMed
-
- Carman GM, Henry SA. Phospholipid biosynthesis in the yeast Saccharomyces cerevisiae and interrelationship with other metabolic processes. Prog Lipid Res. 1999;38:361–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

