FRET measurements of kinesin neck orientation reveal a structural basis for processivity and asymmetry
- PMID: 20212149
- PMCID: PMC2851760
- DOI: 10.1073/pnas.0914924107
FRET measurements of kinesin neck orientation reveal a structural basis for processivity and asymmetry
Abstract
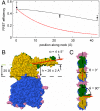
As the smallest and simplest motor enzymes, kinesins have served as the prototype for understanding the relationship between protein structure and mechanochemical function of enzymes in this class. Conventional kinesin (kinesin-1) is a motor enzyme that transports cargo toward the plus end of microtubules by a processive, asymmetric hand-over-hand mechanism. The coiled-coil neck domain, which connects the two kinesin motor domains, contributes to kinesin processivity (the ability to take many steps in a row) and is proposed to be a key determinant of the asymmetry in the kinesin mechanism. While previous studies have defined the orientation and position of microtubule-bound kinesin motor domains, the disposition of the neck coiled-coil remains uncertain. We determined the neck coiled-coil orientation using a multidonor fluorescence resonance energy transfer (FRET) technique to measure distances between microtubules and bound kinesin molecules. Microtubules were labeled with a new fluorescent taxol donor, TAMRA-X-taxol, and kinesin derivatives with an acceptor fluorophore attached at positions on the motor and neck coiled-coil domains were used to reconstruct the positions and orientations of the domains. FRET measurements to positions on the motor domain were largely consistent with the domain orientation determined in previous studies, validating the technique. Measurements to positions on the neck coiled-coil were inconsistent with a radial orientation and instead demonstrated that the neck coiled-coil is parallel to the microtubule surface. The measured orientation provides a structural explanation for how neck surface residues enhance processivity and suggests a simple hypothesis for the origin of kinesin step asymmetry and "limping."
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Family-specific Kinesin Structures Reveal Neck-linker Length Based on Initiation of the Coiled-coil.J Biol Chem. 2016 Sep 23;291(39):20372-86. doi: 10.1074/jbc.M116.737577. Epub 2016 Jul 26. J Biol Chem. 2016. PMID: 27462072 Free PMC article.
-
Role of the kinesin neck region in processive microtubule-based motility.J Cell Biol. 1998 Mar 23;140(6):1407-16. doi: 10.1083/jcb.140.6.1407. J Cell Biol. 1998. PMID: 9508773 Free PMC article.
-
Distinguishing inchworm and hand-over-hand processive kinesin movement by neck rotation measurements.Science. 2002 Feb 1;295(5556):844-8. doi: 10.1126/science.1063089. Science. 2002. PMID: 11823639
-
Kinesin: a molecular motor with a spring in its step.Proc Biol Sci. 2002 Nov 22;269(1507):2363-71. doi: 10.1098/rspb.2002.2117. Proc Biol Sci. 2002. PMID: 12495505 Free PMC article. Review.
-
Kinesins and microtubules: their structures and motor mechanisms.Curr Opin Cell Biol. 2000 Feb;12(1):35-41. doi: 10.1016/s0955-0674(99)00054-x. Curr Opin Cell Biol. 2000. PMID: 10679355 Review.
Cited by
-
Competing instabilities reveal how to rationally design and control active crosslinked gels.Nat Commun. 2022 Oct 29;13(1):6465. doi: 10.1038/s41467-022-34089-9. Nat Commun. 2022. PMID: 36309493 Free PMC article.
-
Topological structure and dynamics of three-dimensional active nematics.Science. 2020 Mar 6;367(6482):1120-1124. doi: 10.1126/science.aaz4547. Science. 2020. PMID: 32139540 Free PMC article.
-
ATP-independent control of autotransporter virulence protein transport via the folding properties of the secreted protein.Chem Biol. 2012 Feb 24;19(2):287-96. doi: 10.1016/j.chembiol.2011.11.009. Epub 2011 Dec 29. Chem Biol. 2012. PMID: 22209629 Free PMC article.
-
Tunable dynamics of microtubule-based active isotropic gels.Philos Trans A Math Phys Eng Sci. 2014 Nov 28;372(2029):20140142. doi: 10.1098/rsta.2014.0142. Philos Trans A Math Phys Eng Sci. 2014. PMID: 25332391 Free PMC article.
-
In Vitro FRET- and Fluorescence-Based Assays to Study Protein Conformation and Protein-Protein Interactions in Mitosis.Methods Mol Biol. 2020;2101:93-122. doi: 10.1007/978-1-0716-0219-5_7. Methods Mol Biol. 2020. PMID: 31879900 Free PMC article.
References
-
- Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–696. - PubMed
-
- Yildiz A, Tomishige M, Vale RD, Selvin PR. Kinesin walks hand-over-hand. Science. 2004;303:676–678. - PubMed
-
- Kaseda K, Higuchi H, Hirose K. Alternate fast and slow stepping of a heterodimeric kinesin molecule. Nat Cell Biol. 2003;5:1079–1082. - PubMed
-
- Hua W, Chung J, Gelles J. Distinguishing inchworm and hand-over-hand processive kinesin movement by neck rotation measurements. Science. 2002;295:844–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

