Monomeric red fluorescent proteins with a large Stokes shift
- PMID: 20212155
- PMCID: PMC2851791
- DOI: 10.1073/pnas.0914365107
Monomeric red fluorescent proteins with a large Stokes shift
Abstract
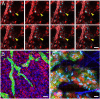
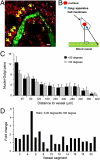
Two-photon microscopy has advanced fluorescence imaging of cellular processes in living animals. Fluorescent proteins in the blue-green wavelength range are widely used in two-photon microscopy; however, the use of red fluorescent proteins is limited by the low power output of Ti-Sapphire lasers above 1,000 nm. To overcome this limitation we have developed two red fluorescent proteins, LSS-mKate1 and LSS-mKate2, which possess large Stokes shifts with excitation/emission maxima at 463/624 and 460/605 nm, respectively. These LSS-mKates are characterized by high pH stability, photostability, rapid chromophore maturation, and monomeric behavior. They lack absorbance in the green region, providing an additional red color to the commonly used red fluorescent proteins. Substantial overlap between the two-photon excitation spectra of the LSS-mKates and blue-green fluorophores enables multicolor imaging using a single laser. We applied this approach to a mouse xenograft model of breast cancer to intravitally study the motility and Golgi-nucleus alignment of tumor cells as a function of their distance from blood vessels. Our data indicate that within 40 mum the breast cancer cells show significant polarization towards vessels in living mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

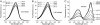

References
-
- Hoffman RM. Imaging cancer dynamics in vivo at the tumor and cellular level with fluorescent proteins. Clin Exp Metastasis. 2009;26:345–355. - PubMed
-
- Wyckoff JB, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. - PubMed
-
- Rubart M. Two-photon microscopy of cells and tissue. Circ Res. 2004;95:1154–1166. - PubMed
-
- Wang W, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62:6278–6288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

