A phase 1 trial of ABT-510 concurrent with standard chemoradiation for patients with newly diagnosed glioblastoma
- PMID: 20212229
- PMCID: PMC6120585
- DOI: 10.1001/archneurol.2010.16
A phase 1 trial of ABT-510 concurrent with standard chemoradiation for patients with newly diagnosed glioblastoma
Abstract
Objective: To determine the maximum tolerated dose of ABT-510, a thrombospondin-1 mimetic drug with antiangiogenic properties, when used concurrently with temozolomide and radiotherapy in patients with newly diagnosed glioblastoma.
Design: Phase 1 dose-escalation clinical trial.
Setting: Comprehensive Cancer Center, University of Alabama at Birmingham. Patients A total of 23 patients with newly diagnosed, histologically verified glioblastoma enrolled between April 2005 and January 2007.
Interventions: Four cohorts of 3 patients each received subcutaneous ABT-510 injection at doses of 20, 50, 100, or 200 mg/d. The maximum cohort was expanded to 14 patients to obtain additional safety and gene expression data. The treatment plan included 10 weeks of induction phase (temozolomide and radiotherapy with ABT-510 for 6 weeks plus ABT-510 monotherapy for 4 weeks) followed by a maintenance phase of ABT-510 and monthly temozolomide.
Main outcome measures: Patients were monitored with brain magnetic resonance imaging and laboratory testing for dose-limiting toxicities, defined as grades 3 or 4 nonhematological toxicities and grade 4 hematological toxicities. Therapy was discontinued if 14 maintenance cycles were completed, disease progression occurred, or if the patient requested withdrawal. Disease progression, survival statistics, and gene expression arrays were analyzed.
Results: There were no grade 3 or 4 dose-limiting toxicity events that appeared related to ABT-510 for the dose range of 20 to 200 mg/d. A maximum tolerated dose was not defined. Most adverse events were mild, and injection-site reactions. The median time to tumor progression was 45.9 weeks, and the median overall survival time was 64.4 weeks. Gene expression analysis using TaqMan low-density arrays identified angiogenic genes that were differentially expressed in the brains of controls compared with patients with newly diagnosed glioblastoma, and identified FGF-1 and TIE-1 as being downregulated in patients who had better clinical outcomes.
Conclusions: ABT-510, at subcutaneous doses up to 200 mg/d, is tolerated well with concurrent temozolomide and radiotherapy in patients with newly diagnosed glioblastoma, and low-density arrays provide a useful method of exploring gene expression profiles.
Figures
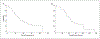



References
-
- Central Brain Tumor Registry of the United States. Statistical Report: Primary Brain Tumors in the United States, 2000-2004. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2008.
-
- Stupp R, Mason WP, van den Bent MJ, et al.; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352(10):987–996. - PubMed
-
- Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992; 359(6398):845–848. - PubMed
-
- Vredenburgh JJ, Desjardins A, Herndon JE II, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4): 1253–1259. - PubMed
-
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

