Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: role of estrogen receptor-alpha versus estrogen receptor-beta
- PMID: 20212268
- PMCID: PMC2876348
- DOI: 10.1161/HYPERTENSIONAHA.109.146399
Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: role of estrogen receptor-alpha versus estrogen receptor-beta
Abstract
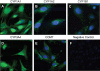
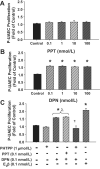
Estradiol-17beta (E(2)beta) and its metabolites, which are sequentially synthesized by cytochrome P450s and catechol-O-methyltransferase to form 2 and 4-hydroxyestradiol (OHE(2)) and 2- and 4-methoxestradiol (ME(2)), are elevated during pregnancy. We investigated whether cytochrome P450s and catechol-O-methyltransferase are expressed in uterine artery endothelial cells (UAECs) and whether E(2)beta and its metabolites modulate cell proliferation via ER-alpha and/or ER-beta and play roles in physiological uterine angiogenesis during pregnancy. Cultured ovine UAECs from pregnant and nonpregnant ewes were treated with 0.1 to 100.0 nmol/L of E(2)beta, 2-OHE(2), 4-OHE(2), 2-ME(2), and 4-ME(2). ER-alpha or ER-beta specificity was tested using ICI 182 780, ER-alpha-specific 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinyleth oxy)phenol]-1H-pyrazole dihydrochloride, ER-beta-specific 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo [1,5-a]pyrim idin-3-yl]phenol antagonists and their respective agonists ER-alpha-specific 4,4',4"-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol and ER-beta-specific 2,3-bis(4-Hydroxyphenyl)-propionitrile. Angiogenesis was evaluated using 5-bromodeoxyuridine proliferation assay. Using confocal microscopy and Western analyses to determine enzyme location and levels, we observed CYP1A1, CYP1A2, CYP1B1, CYP3A4, and catechol-O-methyltransferase expression in UAECs; however, expressions were similar between nonpregnant UAECs and pregnant UAECs. E(2)beta, 2-OHE(2), 4-OHE(2), and 4-ME(2) treatments concentration-dependently stimulated proliferation in pregnant UAECs but not in nonpregnant UAECs; 2-ME(2) did not stimulate proliferation in either cell type. Proliferative responses of pregnant UAECs to E(2)beta were solely mediated by ER-beta, whereas responses to E(2)beta metabolites were neither ER-alpha nor ER-beta mediated. We demonstrate an important vascular role for E(2)beta, its cytochrome P450- and catechol-O-methyltransferase-derived metabolites, and ER-beta in uterine angiogenesis regulation during pregnancy that may be dysfunctional in preeclampsia and other cardiovascular disorders.
Figures





Similar articles
-
Identification of Differential ER-Alpha Versus ER-Beta Mediated Activation of eNOS in Ovine Uterine Artery Endothelial Cells.Biol Reprod. 2016 Jun;94(6):139. doi: 10.1095/biolreprod.115.137554. Epub 2016 May 11. Biol Reprod. 2016. PMID: 27170438 Free PMC article.
-
Estradiol-17β and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites selectively stimulate production of prostacyclin in uterine artery endothelial cells: role of estrogen receptor-α versus estrogen receptor-β.Hypertension. 2013 Feb;61(2):509-18. doi: 10.1161/HYPERTENSIONAHA.112.200717. Epub 2013 Jan 14. Hypertension. 2013. PMID: 23319543 Free PMC article.
-
Effects of the Catechol and Methoxy Metabolites of 17β-Estradiol on Nitric Oxide Production by Ovine Uterine Artery Endothelial Cells.Reprod Sci. 2019 Apr;26(4):459-468. doi: 10.1177/1933719118783265. Epub 2018 Jun 21. Reprod Sci. 2019. PMID: 29929429 Free PMC article.
-
A novel role for an endothelial adrenergic receptor system in mediating catecholestradiol-induced proliferation of uterine artery endothelial cells.Hypertension. 2011 Nov;58(5):874-81. doi: 10.1161/HYPERTENSIONAHA.111.178046. Epub 2011 Sep 26. Hypertension. 2011. PMID: 21947467 Free PMC article.
-
2-Methoxyestradiol, an endogenous 17β-estradiol metabolite, inhibits microglial proliferation and activation via an estrogen receptor-independent mechanism.Am J Physiol Endocrinol Metab. 2016 Mar 1;310(5):E313-22. doi: 10.1152/ajpendo.00418.2015. Epub 2016 Jan 5. Am J Physiol Endocrinol Metab. 2016. PMID: 26732685 Free PMC article.
Cited by
-
Domain-Specific Partitioning of Uterine Artery Endothelial Connexin43 and Caveolin-1.Hypertension. 2016 Oct;68(4):982-8. doi: 10.1161/HYPERTENSIONAHA.116.08000. Epub 2016 Aug 29. Hypertension. 2016. PMID: 27572151 Free PMC article.
-
Identification of Differential ER-Alpha Versus ER-Beta Mediated Activation of eNOS in Ovine Uterine Artery Endothelial Cells.Biol Reprod. 2016 Jun;94(6):139. doi: 10.1095/biolreprod.115.137554. Epub 2016 May 11. Biol Reprod. 2016. PMID: 27170438 Free PMC article.
-
Association between cytochrome P450 1A1 MspI polymorphism and endometrial cancer risk: a meta-analysis.Tumour Biol. 2013 Oct;34(5):2545-50. doi: 10.1007/s13277-013-0798-8. Epub 2013 Aug 7. Tumour Biol. 2013. PMID: 23918309
-
New Lipid Mediators in Retinal Angiogenesis and Retinopathy.Front Pharmacol. 2019 Jul 5;10:739. doi: 10.3389/fphar.2019.00739. eCollection 2019. Front Pharmacol. 2019. PMID: 31333461 Free PMC article. Review.
-
Redox-Sensitive Transcription Factor NRF2 Enhances Trophoblast Differentiation via Induction of miR-1246 and Aromatase.Endocrinology. 2018 May 1;159(5):2022-2033. doi: 10.1210/en.2017-03024. Endocrinology. 2018. PMID: 29546425 Free PMC article.
References
-
- Magness RR. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy. In: Bazer F, editor. The Endocrinology of Pregnancy. Humana press Inc; Totowa, NJ: 1998. pp. 507–539.
-
- Samadi AR, Mayberry RM, Zaidi AA, Pleasant JC, McGhee N, Jr., Rice RJ. Maternal hypertension and associated pregnancy complications among African-American and other women in the United States. Obstet Gynecol. 1996;87:557–563. - PubMed
-
- Pipkin FB. Risk Factors for Preeclampsia. N Engl J Med. 2001;344:925–926. - PubMed
-
- Luft FC. Pieces of the preeclampsia puzzle. Nephrol Dial Transplant. 2003 Nov;18:2209–2210. - PubMed
-
- Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocr Rev. 1990;11:124–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

