Electrochemistry in Media of Exceptionally Low Polarity: Voltammetry with a Fluorous Solvent
- PMID: 20212920
- PMCID: PMC2832752
- DOI: 10.1016/j.jelechem.2009.12.006
Electrochemistry in Media of Exceptionally Low Polarity: Voltammetry with a Fluorous Solvent
Abstract
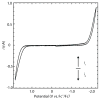
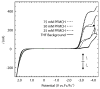
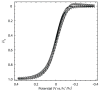
This work demonstrates the first cyclic voltammetry in a perfluorocarbon solvent without use of a cosolvent. The novel electrolyte tetrabutylammonium tetrakis[3,5-bis(perfluorohexyl)phenyl]borate (NBu(4)BArF(104); 80 mM) allows for voltammetry of ferrocene in perfluoro(methylcyclohexane) by lowering the specific resistance to Ω268 k cm at 20.8 °C. Despite significant solution resistance, the resulting voltammograms can be fitted quantitatively without difficulty. The thus determined standard electron transfer rate constant, k°, for the oxidation of ferrocene in perfluoro(methylcyclohexane) is somewhat smaller than for many solvents commonly used in electrochemistry, but can be explained readily as the result of the viscosity and size of the solvent using Marcus theory. Dielectric dispersion spectroscopy verifies that addition of NBu(4)BArF(104) does not significantly raise the overall polarity of the solution over that of neat perfluoro(methylcyclohexane).
Figures





Similar articles
-
Comparison of the conductivity properties of the tetrabutylammonium salt of tetrakis(pentafluorophenyl)borate anion with those of traditional supporting electrolyte anions in nonaqueous solvents.Anal Chem. 2004 Nov 1;76(21):6395-401. doi: 10.1021/ac040087x. Anal Chem. 2004. PMID: 15516133
-
Improved Electrochemistry in Low-Polarity Media Using Tetrakis(pentafluorophenyl)borate Salts as Supporting Electrolytes.Angew Chem Int Ed Engl. 2000 Jan;39(1):248-250. doi: 10.1002/(sici)1521-3773(20000103)39:1<248::aid-anie248>3.0.co;2-3. Angew Chem Int Ed Engl. 2000. PMID: 10649391
-
Ion-Transfer Voltammetry at Fluorous Ether | Water Interfaces.Anal Sci. 2021 Oct 10;37(10):1379-1383. doi: 10.2116/analsci.21P041. Epub 2021 Mar 12. Anal Sci. 2021. PMID: 33716263
-
Application of Ionic Liquids in Electrochemistry-Recent Advances.Molecules. 2020 Dec 9;25(24):5812. doi: 10.3390/molecules25245812. Molecules. 2020. PMID: 33317199 Free PMC article. Review.
-
Recent Advances in Voltammetry.ChemistryOpen. 2015 Jun;4(3):224-60. doi: 10.1002/open.201500042. Epub 2015 May 20. ChemistryOpen. 2015. PMID: 26246984 Free PMC article. Review.
Cited by
-
Impact of Ligand Substitutions on Multielectron Redox Properties of Fe Complexes Supported by Nitrogenous Chelates.ACS Omega. 2018 Nov 2;3(11):14766-14778. doi: 10.1021/acsomega.8b01921. eCollection 2018 Nov 30. ACS Omega. 2018. PMID: 31458151 Free PMC article.
References
-
- Izutsu K. Nonaqueous Solutions. Wiley-VCH; Weinheim: 2002. Electrochemistry.
-
- Ohrenberg C, Geiger WE. Inorg Chem. 2000;39:2948–2950. - PubMed
-
- Hill MG, Lamanna WM, Mann KR. Inorg Chem. 1991;30:4687–4690.
-
- Abbott AP, Claxton TA, Fawcett J, Harper JC. J Chem Soc Faraday Trans. 1996;92:1747–1749.
-
- Geng L, Ewing AG, Jernigan JC, Murray RW. Anal Chem. 1986;58:852–860.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
