Electrochemistry in Media of Exceptionally Low Polarity: Voltammetry with a Fluorous Solvent
- PMID: 20212920
- PMCID: PMC2832752
- DOI: 10.1016/j.jelechem.2009.12.006
Electrochemistry in Media of Exceptionally Low Polarity: Voltammetry with a Fluorous Solvent
Abstract
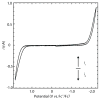
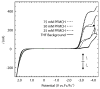
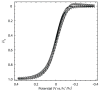
This work demonstrates the first cyclic voltammetry in a perfluorocarbon solvent without use of a cosolvent. The novel electrolyte tetrabutylammonium tetrakis[3,5-bis(perfluorohexyl)phenyl]borate (NBu(4)BArF(104); 80 mM) allows for voltammetry of ferrocene in perfluoro(methylcyclohexane) by lowering the specific resistance to Ω268 k cm at 20.8 °C. Despite significant solution resistance, the resulting voltammograms can be fitted quantitatively without difficulty. The thus determined standard electron transfer rate constant, k°, for the oxidation of ferrocene in perfluoro(methylcyclohexane) is somewhat smaller than for many solvents commonly used in electrochemistry, but can be explained readily as the result of the viscosity and size of the solvent using Marcus theory. Dielectric dispersion spectroscopy verifies that addition of NBu(4)BArF(104) does not significantly raise the overall polarity of the solution over that of neat perfluoro(methylcyclohexane).
Figures





References
-
- Izutsu K. Nonaqueous Solutions. Wiley-VCH; Weinheim: 2002. Electrochemistry.
-
- Ohrenberg C, Geiger WE. Inorg Chem. 2000;39:2948–2950. - PubMed
-
- Hill MG, Lamanna WM, Mann KR. Inorg Chem. 1991;30:4687–4690.
-
- Abbott AP, Claxton TA, Fawcett J, Harper JC. J Chem Soc Faraday Trans. 1996;92:1747–1749.
-
- Geng L, Ewing AG, Jernigan JC, Murray RW. Anal Chem. 1986;58:852–860.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
