Copper binds the carboxy-terminus of trefoil protein 1 (TFF1), favoring its homodimerization and motogenic activity
- PMID: 20213275
- PMCID: PMC11115634
- DOI: 10.1007/s00018-010-0309-7
Copper binds the carboxy-terminus of trefoil protein 1 (TFF1), favoring its homodimerization and motogenic activity
Abstract
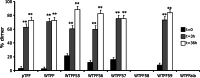
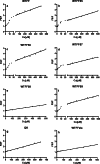
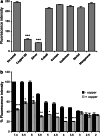
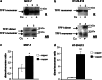
Trefoil protein 1 (TFF1) is a small secreted protein belonging to the trefoil factor family of proteins, that are present mainly in the gastrointestinal (GI) tract and play pivotal roles as motogenic factors in epithelial restitution, cell motility, and other incompletely characterized biological processes. We previously reported the up-regulation of TFF1 gene in copper deficient rats and the unexpected property of the peptide to selectively bind copper. Following the previous evidence, here we report the characterization of the copper binding site by fluorescence quenching spectroscopy and mass spectrometric analyses. We demonstrate that Cys58 and at least three Glu surrounding residues surrounding it, are essential to efficiently bind copper. Moreover, copper binding promotes the TFF1 homodimerization, thus increasing its motogenic activity in in vitro wound healing assays. Copper levels could then modulate the TFF1 functions in the GI tract, as well as its postulated role in cancer progression and invasion.
Figures


Similar articles
-
Copper promotes TFF1-mediated Helicobacter pylori colonization.PLoS One. 2013 Nov 13;8(11):e79455. doi: 10.1371/journal.pone.0079455. eCollection 2013. PLoS One. 2013. PMID: 24236136 Free PMC article.
-
Interaction between TFF1, a gastric tumor suppressor trefoil protein, and TFIZ1, a brichos domain-containing protein with homology to SP-C.Biochemistry. 2005 Jun 7;44(22):7967-75. doi: 10.1021/bi047287n. Biochemistry. 2005. PMID: 15924415
-
Trefoil Factor 1 is involved in gastric cell copper homeostasis.Int J Biochem Cell Biol. 2015 Feb;59:30-40. doi: 10.1016/j.biocel.2014.11.014. Epub 2014 Dec 5. Int J Biochem Cell Biol. 2015. PMID: 25486181
-
[Trefoil factor 1 (pS2/TFF1), a peptide with numerous functions].Bull Cancer. 2005 Sep;92(9):773-81. Bull Cancer. 2005. PMID: 16203267 Review. French.
-
Trefoil Factor Family (TFF) Peptides and Their Diverse Molecular Functions in Mucus Barrier Protection and More: Changing the Paradigm.Int J Mol Sci. 2020 Jun 25;21(12):4535. doi: 10.3390/ijms21124535. Int J Mol Sci. 2020. PMID: 32630599 Free PMC article. Review.
Cited by
-
The Tumor Suppressor TFF1 Occurs in Different Forms and Interacts with Multiple Partners in the Human Gastric Mucus Barrier: Indications for Diverse Protective Functions.Int J Mol Sci. 2020 Apr 4;21(7):2508. doi: 10.3390/ijms21072508. Int J Mol Sci. 2020. PMID: 32260357 Free PMC article.
-
Molecular Alterations in the Stomach of Tff1-Deficient Mice: Early Steps in Antral Carcinogenesis.Int J Mol Sci. 2020 Jan 18;21(2):644. doi: 10.3390/ijms21020644. Int J Mol Sci. 2020. PMID: 31963721 Free PMC article.
-
Gastric TFF1 Expression from Acute to Chronic Helicobacter Infection.Front Cell Infect Microbiol. 2017 Oct 9;7:434. doi: 10.3389/fcimb.2017.00434. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29085807 Free PMC article.
-
Copper promotes TFF1-mediated Helicobacter pylori colonization.PLoS One. 2013 Nov 13;8(11):e79455. doi: 10.1371/journal.pone.0079455. eCollection 2013. PLoS One. 2013. PMID: 24236136 Free PMC article.
-
Nutritional Aspects of Gastrointestinal Wound Healing.Adv Wound Care (New Rochelle). 2016 Nov 1;5(11):507-515. doi: 10.1089/wound.2015.0671. Adv Wound Care (New Rochelle). 2016. PMID: 27867755 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

