High density lipoprotein structure-function and role in reverse cholesterol transport
- PMID: 20213545
- PMCID: PMC3215094
- DOI: 10.1007/978-90-481-8622-8_7
High density lipoprotein structure-function and role in reverse cholesterol transport
Abstract
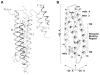
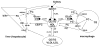
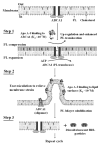
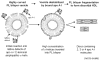
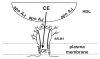
High density lipoprotein (HDL) possesses important anti-atherogenic properties and this review addresses the molecular mechanisms underlying these functions. The structures and cholesterol transport abilities of HDL particles are determined by the properties of their exchangeable apolipoprotein (apo) components. ApoA-I and apoE, which are the best characterized in structural terms, contain a series of amphipathic alpha-helical repeats. The helices located in the amino-terminal two-thirds of the molecule adopt a helix bundle structure while the carboxy-terminal segment forms a separately folded, relatively disorganized, domain. The latter domain initiates lipid binding and this interaction induces changes in conformation; the alpha-helix content increases and the amino-terminal helix bundle can open subsequently. These conformational changes alter the abilities of apoA-I and apoE to function as ligands for their receptors. The apoA-I and apoE molecules possess detergent-like properties and they can solubilize vesicular phospholipid to create discoidal HDL particles with hydrodynamic diameters of ~10 nm. In the case of apoA-I, such a particle is stabilized by two protein molecules arranged in an anti-parallel, double-belt, conformation around the edge of the disc. The abilities of apoA-I and apoE to solubilize phospholipid and stabilize HDL particles enable these proteins to be partners with ABCA1 in mediating efflux of cellular phospholipid and cholesterol, and the biogenesis of HDL particles. ApoA-I-containing nascent HDL particles play a critical role in cholesterol transport in the circulation whereas apoE-containing HDL particles mediate cholesterol transport in the brain. The mechanisms by which HDL particles are remodeled by lipases and lipid transfer proteins, and interact with SR-BI to deliver cholesterol to cells, are reviewed.
Figures

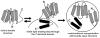

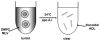

Similar articles
-
New insights into the determination of HDL structure by apolipoproteins: Thematic review series: high density lipoprotein structure, function, and metabolism.J Lipid Res. 2013 Aug;54(8):2034-2048. doi: 10.1194/jlr.R034025. Epub 2012 Dec 10. J Lipid Res. 2013. PMID: 23230082 Free PMC article. Review.
-
Influence of apolipoprotein (Apo) A-I structure on nascent high density lipoprotein (HDL) particle size distribution.J Biol Chem. 2010 Oct 15;285(42):31965-73. doi: 10.1074/jbc.M110.126292. Epub 2010 Aug 2. J Biol Chem. 2010. PMID: 20679346 Free PMC article.
-
Influence of C-terminal α-helix hydrophobicity and aromatic amino acid content on apolipoprotein A-I functionality.Biochim Biophys Acta. 2012 Mar;1821(3):456-63. doi: 10.1016/j.bbalip.2011.07.020. Epub 2011 Aug 5. Biochim Biophys Acta. 2012. PMID: 21840419 Free PMC article.
-
[Role of apolipoprotein AI on nascent high-density lipoprotein particle formation].Sheng Li Ke Xue Jin Zhan. 2014 Apr;45(2):81-6. Sheng Li Ke Xue Jin Zhan. 2014. PMID: 25069300 Chinese.
-
Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL.J Mol Med (Berl). 2006 Apr;84(4):276-94. doi: 10.1007/s00109-005-0030-4. Epub 2006 Feb 25. J Mol Med (Berl). 2006. PMID: 16501936 Review.
Cited by
-
Molecular mechanisms responsible for the differential effects of apoE3 and apoE4 on plasma lipoprotein-cholesterol levels.Arterioscler Thromb Vasc Biol. 2013 Apr;33(4):687-93. doi: 10.1161/ATVBAHA.112.301193. Epub 2013 Feb 14. Arterioscler Thromb Vasc Biol. 2013. PMID: 23413428 Free PMC article.
-
Folded functional lipid-poor apolipoprotein A-I obtained by heating of high-density lipoproteins: relevance to high-density lipoprotein biogenesis.Biochem J. 2012 Mar 15;442(3):703-12. doi: 10.1042/BJ20111831. Biochem J. 2012. PMID: 22150513 Free PMC article.
-
Engineered rHDL Nanoparticles as a Suitable Platform for Theranostic Applications.Molecules. 2022 Oct 19;27(20):7046. doi: 10.3390/molecules27207046. Molecules. 2022. PMID: 36296638 Free PMC article.
-
High HDL (High-Density Lipoprotein) Cholesterol Increases Cardiovascular Risk in Hypertensive Patients.Hypertension. 2022 Oct;79(10):2355-2363. doi: 10.1161/HYPERTENSIONAHA.122.19912. Epub 2022 Aug 15. Hypertension. 2022. PMID: 35968698 Free PMC article.
-
Caloric restriction or telmisartan control dyslipidemia and nephropathy in obese diabetic Zücker rats.Diabetol Metab Syndr. 2014 Jan 27;6(1):10. doi: 10.1186/1758-5996-6-10. Diabetol Metab Syndr. 2014. PMID: 24468233 Free PMC article.
References
-
- Abe-Dohmae S, Kato KH, Kumon Y, Hu W, Ishigami H, Iwamoto N, Okazaki M, Wu CA, Ueda K, Yokoyama S. Serum amyloid A generates high density lipoprotein with cellular lipid in an ABCA1- or ABCA7-dependent manner. J Lipid Res. 2006;47:1542–1550. - PubMed
-
- Acharya P, Segall ML, Zaiou M, Morrow JA, Weisgraber K, Phillips MC, Lund-Katz S, Snow JW. Comparison of the stabilities and unfolding pathways of human apolipoprotein E isoforms by differential scanning calorimetry and circular dichroism. Biochim Biophys Acta. 2002;1584:9–19. - PubMed
-
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. - PubMed
-
- Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. - PubMed
-
- Aggerbeck LP, Wetterau JR, Weisgraber KH, Wu CSC, Lindgren FT. Human apolipoprotein E3 in aqueous solution. II Properties of the amino- and carboxyl-terminal domains. J Biol Chem. 1988;263:6249–6258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

