Cholesterol and ion channels
- PMID: 20213557
- PMCID: PMC2895485
- DOI: 10.1007/978-90-481-8622-8_19
Cholesterol and ion channels
Abstract
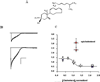
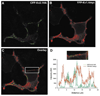
A variety of ion channels, including members of all major ion channel families, have been shown to be regulated by changes in the level of membrane cholesterol and partition into cholesterol-rich membrane domains. In general, several types of cholesterol effects have been described. The most common effect is suppression of channel activity by an increase in membrane cholesterol, an effect that was described for several types of inwardly-rectifying K(+) channels, voltage-gated K(+) channels, Ca(+2) sensitive K(+) channels, voltage-gated Na(+) channels, N-type voltage-gated Ca(+2) channels and volume-regulated anion channels. In contrast, several types of ion channels, such as epithelial amiloride-sensitive Na(+) channels and Transient Receptor Potential channels, as well as some of the types of inwardly-rectifying and voltage-gated K(+) channels were shown to be inhibited by cholesterol depletion. Cholesterol was also shown to alter the kinetic properties and current-voltage dependence of several voltage-gated channels. Finally, maintaining membrane cholesterol level is required for coupling ion channels to signalling cascades. In terms of the mechanisms, three general mechanisms have been proposed: (i) specific interactions between cholesterol and the channel protein, (ii) changes in the physical properties of the membrane bilayer and (iii) maintaining the scaffolds for protein-protein interactions. The goal of this review is to describe systematically the role of cholesterol in regulation of the major types of ion channels and to discuss these effects in the context of the models proposed.
Figures


Similar articles
-
The human red cell voltage-regulated cation channel. The interplay with the chloride conductance, the Ca(2+)-activated K(+) channel and the Ca(2+) pump.J Membr Biol. 2003 Sep 1;195(1):1-8. doi: 10.1007/s00232-003-2036-6. J Membr Biol. 2003. PMID: 14502420
-
Inwardly rectifying and voltage-gated outward potassium channels exhibit low sensitivity to methylmercury.Neurotoxicology. 2005 Jun;26(3):439-54. doi: 10.1016/j.neuro.2005.03.005. Epub 2005 Apr 26. Neurotoxicology. 2005. PMID: 15935214
-
Voltage-dependent gating in a "voltage sensor-less" ion channel.PLoS Biol. 2010 Feb 23;8(2):e1000315. doi: 10.1371/journal.pbio.1000315. PLoS Biol. 2010. PMID: 20208975 Free PMC article.
-
Targeting of ion channels to membrane microdomains: localization of KV channels to lipid rafts.Trends Pharmacol Sci. 2004 Jan;25(1):16-21. doi: 10.1016/j.tips.2003.11.007. Trends Pharmacol Sci. 2004. PMID: 14723974 Review.
-
Osteoclast ion channels: potential targets for antiresorptive drugs.Curr Pharm Des. 2001 May;7(8):637-54. doi: 10.2174/1381612013397799. Curr Pharm Des. 2001. PMID: 11375773 Review.
Cited by
-
Plasma membrane protein trafficking in plant-microbe interactions: a plant cell point of view.Front Plant Sci. 2014 Dec 22;5:735. doi: 10.3389/fpls.2014.00735. eCollection 2014. Front Plant Sci. 2014. PMID: 25566303 Free PMC article.
-
Stiffened lipid platforms at molecular force foci.Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):4886-92. doi: 10.1073/pnas.1302018110. Epub 2013 Mar 8. Proc Natl Acad Sci U S A. 2013. PMID: 23476066 Free PMC article. Review.
-
Association Between HDL Cholesterol and QTc Interval: A Population-Based Epidemiological Study.J Clin Med. 2019 Sep 23;8(10):1527. doi: 10.3390/jcm8101527. J Clin Med. 2019. PMID: 31547597 Free PMC article.
-
Inhibition of cardiac pacemaker channel hHCN2 depends on intercalation of lipopolysaccharide into channel-containing membrane microdomains.J Physiol. 2014 Mar 15;592(6):1199-211. doi: 10.1113/jphysiol.2013.268540. Epub 2013 Dec 23. J Physiol. 2014. PMID: 24366264 Free PMC article.
-
Amiodarone Alters Cholesterol Biosynthesis through Tissue-Dependent Inhibition of Emopamil Binding Protein and Dehydrocholesterol Reductase 24.ACS Chem Neurosci. 2020 May 20;11(10):1413-1423. doi: 10.1021/acschemneuro.0c00042. Epub 2020 Apr 29. ACS Chem Neurosci. 2020. PMID: 32286791 Free PMC article.
References
-
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. - PubMed
-
- Babiychuk EB, Smith RD, Burdyga T, Babiychuk VS, Wray S, Draeger A. Membrane cholesterol regulates smooth muscle phasic contraction. J Membr Biol. 2004;198:95–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

