Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation
- PMID: 20213734
- PMCID: PMC2944234
- DOI: 10.1002/eji.200939455
Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation
Abstract
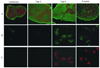
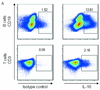
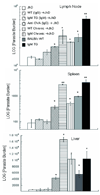
In visceral leishmaniasis, the draining LN (DLN) is the initial site for colonization and establishment of infection after intradermal transmission by the sand fly vector; however, little is known about the developing immune response within this site. Using an intradermal infection model, which allows for parasite visceralization, we have examined the ongoing immune responses in the DLN of BALB/c mice infected with Leishmania infantum. Although not unexpected, at early times post-infection there is a marked B-cell expansion in the DLN, which persists throughout infection. However, the characteristics of this response were of interest; as early as day 7 post-infection, polyclonal antibodies (TNP, OVA, chromatin) were observed and the levels appeared comparable to the specific anti-leishmania response. Although B-cell-deficient JhD BALB/c mice are relatively resistant to infection, neither B-cell-derived IL-10 nor B-cell antigen presentation appear to be primarily responsible for the elevated parasitemia. However, passive transfer and reconstitution of JhD BALB/c with secretory immunoglobulins, (IgM or IgG; specific or non-specific immune complexes) results in increased susceptibility to L. infantum infection. Further, JhD BALB/c mice transgenetically reconstituted to secrete IgM demonstrated exacerbated disease in comparison to WT BALB/c mice as early as 2 days post-infection. Evidence suggests that complement activation (generation of C5a) and signaling via the C5a receptor (CD88) is related to the disease exacerbation caused by IgM rather than cytokine levels (IL-10 or IFN-gamma). Overall these studies indicate that polyclonal B-cell activation, which is known to be associated with human visceral leishmaniasis, is an early and intrinsic characteristic of disease and may represent a target for therapeutic intervention.
Conflict of interest statement
Figures

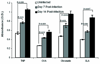

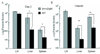
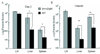

References
-
- Stanley AC, Engwerda CR. Balancing immunity and pathology in visceral leishmaniasis. Immunol Cell Biol. 2007;85:138–147. - PubMed
-
- Kaye PM, Svensson M, Ato M, Maroof A, Polley R, Stager S, Zubairi S, Engwerda CR. The immunopathology of experimental visceral leishmaniasis. Immunol Rev. 2004;201:239–253. - PubMed
-
- Mueller SN, Ahmed R. Lymphoid stroma in the initiation and control of immune responses. Immunol Rev. 2008;224:284–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI027811/AI/NIAID NIH HHS/United States
- T32 AI07404/AI/NIAID NIH HHS/United States
- R01 AI027811/AI/NIAID NIH HHS/United States
- AI27811/AI/NIAID NIH HHS/United States
- R01 GM062134/GM/NIGMS NIH HHS/United States
- T32 AI007404/AI/NIAID NIH HHS/United States
- R01 AI045044/AI/NIAID NIH HHS/United States
- AI45044/AI/NIAID NIH HHS/United States
- R56 AI043603/AI/NIAID NIH HHS/United States
- AI-068730/AI/NIAID NIH HHS/United States
- AI-43603/AI/NIAID NIH HHS/United States
- GM-62134/GM/NIGMS NIH HHS/United States
- P01 AI068730/AI/NIAID NIH HHS/United States
- R01 AI043603/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

