Mice lacking C1q or C3 show accelerated rejection of minor H disparate skin grafts and resistance to induction of tolerance
- PMID: 20213737
- PMCID: PMC2988415
- DOI: 10.1002/eji.200940158
Mice lacking C1q or C3 show accelerated rejection of minor H disparate skin grafts and resistance to induction of tolerance
Abstract
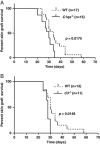
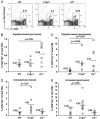
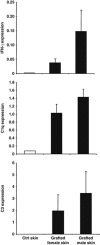
Complement activation is known to have deleterious effects on organ transplantation. On the other hand, the complement system is also known to have an important role in regulating immune responses. The balance between these two opposing effects is critical in the context of transplantation. Here, we report that female mice deficient in C1q (C1qa(-/-)) or C3 (C3(-/-)) reject male syngeneic grafts (HY incompatible) at an accelerated rate compared with WT mice. Intranasal HY peptide administration, which induces tolerance to syngeneic male grafts in WT mice, fails to induce tolerance in C1qa(-/-) or C3(-/-) mice. The rejection of the male grafts correlated with the presence of HY D(b)Uty-specific CD8(+) T cells. Consistent with this, peptide-treated C1qa(-/-) and C3(-/-) female mice rejecting male grafts exhibited more antigen-specific CD8(+)IFN-gamma(+) and CD8(+)IL-10(+) cells compared with WT females. This suggests that accumulation of IFN-gamma- and IL-10-producing T cells may play a key role in mediating the ongoing inflammatory process and graft rejection. Interestingly, within the tolerized male skin grafts of peptide-treated WT mice, IFN-gamma, C1q and C3 mRNA levels were higher compared to control female grafts. These results suggest that C1q and C3 facilitate the induction of intranasal tolerance.
Figures


Similar articles
-
Transplantation tolerance induced by intranasal administration of HY peptides.Blood. 2004 May 15;103(10):3951-9. doi: 10.1182/blood-2003-11-3763. Epub 2004 Jan 15. Blood. 2004. PMID: 14726386
-
DNA fusion vaccines induce targeted epitope-specific CTLs against minor histocompatibility antigens from a normal or tolerized repertoire.J Immunol. 2004 Oct 1;173(7):4492-9. doi: 10.4049/jimmunol.173.7.4492. J Immunol. 2004. PMID: 15383580
-
Molecular mechanisms of induction of antigen-specific allograft tolerance by intranasal peptide administration.J Immunol. 2011 May 15;186(10):5719-28. doi: 10.4049/jimmunol.1002444. Epub 2011 Apr 13. J Immunol. 2011. PMID: 21490154 Free PMC article.
-
A continuous infusion of a minor histocompatibility antigen-immunodominant peptide induces a delay of male skin graft rejection.Immunobiology. 2009;214(8):703-11. doi: 10.1016/j.imbio.2008.12.004. Epub 2009 Feb 26. Immunobiology. 2009. PMID: 19249121
-
Intranasal peptide-induced tolerance and linked suppression: consequences of complement deficiency.Immunology. 2015 Jan;144(1):149-57. doi: 10.1111/imm.12358. Immunology. 2015. PMID: 25039245 Free PMC article.
Cited by
-
Genetic variants in the region of the C1q genes are associated with rheumatoid arthritis.Clin Exp Immunol. 2013 Jul;173(1):76-83. doi: 10.1111/cei.12097. Clin Exp Immunol. 2013. PMID: 23607884 Free PMC article.
-
Recent advances into the role of pattern recognition receptors in transplantation.Cell Immunol. 2020 May;351:104088. doi: 10.1016/j.cellimm.2020.104088. Epub 2020 Mar 7. Cell Immunol. 2020. PMID: 32183988 Free PMC article. Review.
-
C1q as a target molecule to treat human disease: What do mouse studies teach us?Front Immunol. 2022 Aug 3;13:958273. doi: 10.3389/fimmu.2022.958273. eCollection 2022. Front Immunol. 2022. PMID: 35990646 Free PMC article.
-
Annexin A2 and A5 serve as new ligands for C1q on apoptotic cells.J Biol Chem. 2012 Sep 28;287(40):33733-44. doi: 10.1074/jbc.M112.341339. Epub 2012 Aug 9. J Biol Chem. 2012. PMID: 22879587 Free PMC article.
-
Genome-Wide CRISPR Screen Identifies Host Factors Required by Toxoplasma gondii Infection.Front Cell Infect Microbiol. 2020 Jan 22;9:460. doi: 10.3389/fcimb.2019.00460. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 32039045 Free PMC article.
References
-
- Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat. Rev. Immunol. 2007;7:9–18. - PubMed
-
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. - PubMed
-
- Baldwin WM, III, Kasper EK, Zachary AA, Wasowska BA, Rodriguez ER. Beyond C4d: other complement-related diagnostic approaches to antibody-mediated rejection. Am. J. Transplant. 2004;4:311–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

