Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis
- PMID: 20213804
- PMCID: PMC3866029
- DOI: 10.1002/art.27382
Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis
Abstract
Objective: Connective tissue growth factor (CTGF) is a cysteine-rich secreted matricellular protein involved in wound healing and tissue repair. Enhanced and prolonged expression of CTGF has been associated with tissue fibrosis in humans. However, questions remain as to whether CTGF expression alone is sufficient to drive fibrosis. This study was undertaken to investigate whether CTGF alone is sufficient to cause fibrosis in intact animals and whether its effects are mediated through activation of transforming growth factor beta (TGFbeta) signaling or through distinct signal transduction pathways.
Methods: We generated mice overexpressing CTGF in fibroblasts under the control of the fibroblast-specific collagen alpha2(I) promoter enhancer. Tissues such as skin, lung, and kidney were harvested for histologic analysis. Mouse embryonic fibroblasts were prepared from embryos (14.5 days postcoitum) for biochemical analysis.
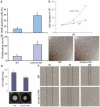
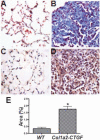
Results: Mice overexpressing CTGF in fibroblasts were susceptible to accelerated tissue fibrosis affecting the skin, lung, kidney, and vasculature, most notably the small arteries. We identified a marked expansion of the myofibroblast cell population in the dermis. RNA analysis of transgenic dermal fibroblasts revealed elevated expression of key matrix genes, consistent with a fibrogenic response. CTGF induced phosphorylation of p38, ERK-1/2, JNK, and Akt, but not Smad3, in transgenic mouse fibroblasts compared with wild-type mouse fibroblasts. Transfection experiments showed significantly increased basal activity of the CTGF and serum response element promoters, and enhanced induction of the CTGF promoter in the presence of TGFbeta.
Conclusion: These results demonstrate that selective expression of CTGF in fibroblasts alone causes tissue fibrosis in vivo through specific signaling pathways, integrating cues from the extracellular matrix into signal transduction pathways to orchestrate pivotal biologic responses relevant to tissue repair and fibrosis.
Figures




References
-
- Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125–30. - PubMed
-
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–10. - PubMed
-
- Friedrichsen S, Heuer H, Christ S, Winckler M, Brauer D, Bauer K, et al. CTGF expression during mouse embryonic development. Cell Tissue Res. 2003;312:175–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

