Constructing kidney-like tissues from cells based on programs for organ development: toward a method of in vitro tissue engineering of the kidney
- PMID: 20214453
- PMCID: PMC2947461
- DOI: 10.1089/ten.TEA.2009.0548
Constructing kidney-like tissues from cells based on programs for organ development: toward a method of in vitro tissue engineering of the kidney
Abstract
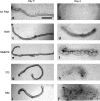
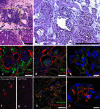
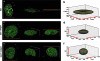
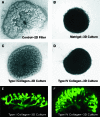
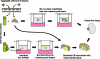
The plausibility of constructing vascularized three-dimensional (3D) kidney tissue from cells was investigated. The kidney develops from mutual inductive interactions between cells of the ureteric bud (UB), derived from the Wolffian duct (WD), and the metanephric mesenchyme (MM). We found that isolated MMs were capable of inducing branching morphogenesis of the WD (an epithelial tube) in recombination cultures; suggesting that the isolated MM retains inductive capacity for WD-derived epithelial tubule cells other than those from the UB. Hanging drop aggregates of embryonic and adult renal epithelial cells from UB and mouse inner medullary collecting duct cell (IMCD) lines, which are ultimately of WD origin, were capable of inducing MM epithelialization and tubulogenesis with apparent connections (UB cells) and collecting duct-like tubules with lumens (IMCD). This supports the view that the collecting system can be constructed from certain epithelial cells (those ultimately of WD origin) when stimulated by MM. Although the functions of the MM could not be replaced by cultured mesenchymal cells, primary MM cells and one MM-derived cell line (BSN) produced factors that stimulate UB branching morphogenesis, whereas another, rat inducible metanephric mesenchyme (RIMM-18), supported WD budding as a feeder layer. This indicates that some MM functions can be recapitulated by cells. Although engineering of a kidney-like tissue from cultured cells alone remains to be achieved, these results suggest the feasibility of such an approach following the normal developmental progression of the UB and MM. Consistent with this notion, implants of kidney-like tissues constructed in vitro from recombinations of the UB and MM survived for over 5 weeks and achieved an apparently host-derived glomerular vasculature. Lastly, we addressed the issue of optimal macro- and micro-patterning of kidney-like tissue, which might be necessary for function of an organ assembled using a tissue engineering approach. To identify suitable conditions, 3D reconstructions of HoxB7-green fluorescent protein mouse rudiments (E12) cultured on a filter or suspended in a collagen gel (type I or type IV) revealed that type IV collagen 3D culture supports the deepest tissue growth (600 +/- 8 microm) and the largest kidney volume (0.22 +/- 0.02 mm(3)), and enabled the development of an umbrella-shaped collecting system such as occurs in vivo. Taken together with prior work (Rosines et al., 2007; Steer et al., 2002), these results support the plausibility of a developmental strategy for constructing and propagating vascularized 3D kidney-like tissues from recombinations of cultured renal progenitor cells and/or primordial tissue.
Figures


Similar articles
-
Concise review: can the intrinsic power of branching morphogenesis be used for engineering epithelial tissues and organs?Stem Cells Transl Med. 2013 Dec;2(12):993-1000. doi: 10.5966/sctm.2013-0076. Epub 2013 Nov 4. Stem Cells Transl Med. 2013. PMID: 24191267 Free PMC article. Review.
-
Development and differentiation of the ureteric bud into the ureter in the absence of a kidney collecting system.Dev Biol. 2006 Oct 15;298(2):571-84. doi: 10.1016/j.ydbio.2006.07.006. Epub 2006 Jul 12. Dev Biol. 2006. PMID: 16934795
-
Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney.Dev Biol. 2004 Nov 1;275(1):44-67. doi: 10.1016/j.ydbio.2004.07.022. Dev Biol. 2004. PMID: 15464572
-
The effect of hyaluronic acid size and concentration on branching morphogenesis and tubule differentiation in developing kidney culture systems: potential applications to engineering of renal tissues.Biomaterials. 2007 Nov;28(32):4806-17. doi: 10.1016/j.biomaterials.2007.07.034. Epub 2007 Aug 15. Biomaterials. 2007. PMID: 17706761 Free PMC article.
-
The origin of the mammalian kidney: implications for recreating the kidney in vitro.Development. 2015 Jun 1;142(11):1937-47. doi: 10.1242/dev.104802. Development. 2015. PMID: 26015537 Review.
Cited by
-
Concise review: can the intrinsic power of branching morphogenesis be used for engineering epithelial tissues and organs?Stem Cells Transl Med. 2013 Dec;2(12):993-1000. doi: 10.5966/sctm.2013-0076. Epub 2013 Nov 4. Stem Cells Transl Med. 2013. PMID: 24191267 Free PMC article. Review.
-
Cell Printing in Complex Hydrogel Scaffolds.IEEE Trans Nanobioscience. 2019 Apr;18(2):265-268. doi: 10.1109/TNB.2019.2905517. Epub 2019 Mar 15. IEEE Trans Nanobioscience. 2019. PMID: 30892231 Free PMC article.
-
Protein kinase A regulates GDNF/RET-dependent but not GDNF/Ret-independent ureteric bud outgrowth from the Wolffian duct.Dev Biol. 2010 Nov 15;347(2):337-47. doi: 10.1016/j.ydbio.2010.08.029. Epub 2010 Sep 15. Dev Biol. 2010. PMID: 20816800 Free PMC article.
-
Complexation-induced resolution enhancement of 3D-printed hydrogel constructs.Nat Commun. 2020 Mar 9;11(1):1267. doi: 10.1038/s41467-020-14997-4. Nat Commun. 2020. PMID: 32152307 Free PMC article.
-
Stem Cells and Regenerative Medicine: Myth or Reality of the 21th Century.Stem Cells Int. 2015;2015:734731. doi: 10.1155/2015/734731. Epub 2015 Aug 2. Stem Cells Int. 2015. PMID: 26300923 Free PMC article. Review.
References
-
- Steer D.L. Bush K.T. Meyer T.N. Schwesinger C. Nigam S.K. A strategy for in vitro propagation of rat nephrons. Kidney Int. 2002;62:1958. - PubMed
-
- OPTN. Annual report of the U.S. Organ procurement and transplantation network (OPTN) and the scientific registry of transplant recipients: Current U.S. Waiting list based on OPTN data as of April 2, 2010. (Dept. of Health and Human Services.) 2010.
-
- Rogers S.A. Hammerman M.R. Transplantation of rat metanephroi into mice. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1865. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

