Oxygen-controlled three-dimensional cultures to analyze tumor angiogenesis
- PMID: 20214469
- PMCID: PMC2947932
- DOI: 10.1089/ten.tea.2009.0670
Oxygen-controlled three-dimensional cultures to analyze tumor angiogenesis
Abstract
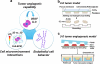
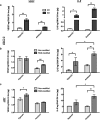
Tumor angiogenesis is controlled by the integrated action of physicochemical and biological cues; however, the individual contributions of these cues are not well understood. We have designed alginate-based microscale tumor models to define the distinct importance of oxygen concentration, culture dimensionality, and cell-extracellular matrix interactions on the angiogenic capability of oral squamous cell carcinoma, and have verified the relevance of our findings with U87 glioblastoma cells. Our results revealed qualitative differences in the microenvironmental regulation of vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) secretion in three-dimensional (3D) culture. Specifically, IL-8 secretion was highest under ambient conditions, whereas VEGF secretion was highest in hypoxic cultures. Additionally, 3D integrin engagement by RGD-modified alginate matrices increased IL-8 secretion independently of oxygen, whereas VEGF secretion was only moderately affected by cell-extracellular matrix interactions. Using two-dimensional migration assays and a new 3D tumor angiogenesis model, we demonstrated that the resulting angiogenic signaling promotes tumor angiogenesis by increasing endothelial cell migration and invasion. Collectively, tissue-engineered tumor models improve our understanding of tumor angiogenesis, which may ultimately advance anticancer therapies.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

