Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis
- PMID: 20214476
- PMCID: PMC2946637
- DOI: 10.1086/651454
Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis
Abstract
Background: South African guidelines recommend protease-inhibitor-based antiretroviral therapy (ART) with lopinavir-ritonavir for human immunodeficiency virus (HIV)-infected children <36 months of age. We investigated factors associated with viral suppression and mortality among young children initiating ART.
Methods: Treatment-naive, ART-eligible, HIV-infected children (aged 6-104 weeks) were enrolled in an ART strategies trial in South Africa and initiated protease-inhibitor-based ART. Mortality and the probability of viral suppression (defined as HIV RNA load of <400 copies/mL) by 39 weeks after ART initiation were investigated.
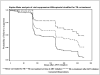
Results: Of 254 children who initiated ART, 99 (39%) were cotreated for tuberculosis during follow-up. The mortality rate was 14%. Factors predicting mortality were lower pre-ART weight-for-age z score and higher HIV RNA load. By 39 weeks, 84% of surviving children achieved viral suppression. Children who were not cotreated for tuberculosis were more likely to achieve viral suppression (94.8%) than were children who were receiving cotreatment at ART initiation (74.2%) or who started tuberculosis cotreatment after ART initiation (51.6%; P < .001). Other factors predicting lower probability of viral suppression were lower pre-ART weight- and length-for-age z score, higher HIV RNA load, and World Health Organization disease stage.
Conclusion: High rates of viral suppression can be achieved among infants and young children who initiate protease-inhibitor-based ART. Cotreatment for tuberculosis reduced viral suppression. How best to treat HIV-infected children who require tuberculosis treatment warrants urgent investigation.
Comment in
-
Antiretroviral therapy in children with tuberculosis: progress toward defining the issues.J Infect Dis. 2010 Apr 15;201(8):1113-4. doi: 10.1086/651455. J Infect Dis. 2010. PMID: 20214477 No abstract available.
Similar articles
-
Antiretroviral therapy responses among children attending a large public clinic in Soweto, South Africa.Pediatr Infect Dis J. 2011 Nov;30(11):974-9. doi: 10.1097/INF.0b013e31822539f6. Pediatr Infect Dis J. 2011. PMID: 21734620 Free PMC article.
-
Switching children previously exposed to nevirapine to nevirapine-based treatment after initial suppression with a protease-inhibitor-based regimen: long-term follow-up of a randomised, open-label trial.Lancet Infect Dis. 2012 Jul;12(7):521-30. doi: 10.1016/S1473-3099(12)70051-8. Epub 2012 Mar 16. Lancet Infect Dis. 2012. PMID: 22424722 Free PMC article. Clinical Trial.
-
Lopinavir exposure is insufficient in children given double doses of lopinavir/ritonavir during rifampicin-based treatment for tuberculosis.Antivir Ther. 2011;16(3):417-21. doi: 10.3851/IMP1757. Antivir Ther. 2011. PMID: 21555825
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
-
[Lopinavir/ritonavir monotherapy as a simplification strategy in antiretroviral therapy in clinical practice].Enferm Infecc Microbiol Clin. 2008 Dec;26 Suppl 16:24-6. doi: 10.1016/s0213-005x(08)76607-2. Enferm Infecc Microbiol Clin. 2008. PMID: 19572441 Review. Spanish.
Cited by
-
Genetic Changes in HIV-1 Gag-Protease Associated with Protease Inhibitor-Based Therapy Failure in Pediatric Patients.AIDS Res Hum Retroviruses. 2015 Aug;31(8):776-82. doi: 10.1089/AID.2014.0349. Epub 2015 Jun 4. AIDS Res Hum Retroviruses. 2015. PMID: 25919760 Free PMC article.
-
Clinical Outcomes in Children With Human Immunodeficiency Virus Treated for Nonsevere Tuberculosis in the SHINE Trial.Clin Infect Dis. 2024 Jul 19;79(1):70-77. doi: 10.1093/cid/ciae193. Clin Infect Dis. 2024. PMID: 38592950 Free PMC article. Clinical Trial.
-
Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial.JAMA. 2010 Sep 8;304(10):1082-90. doi: 10.1001/jama.2010.1278. JAMA. 2010. PMID: 20823434 Free PMC article. Clinical Trial.
-
Growth reconstitution following antiretroviral therapy and nutritional supplementation: systematic review and meta-analysis.AIDS. 2015 Sep 24;29(15):2009-23. doi: 10.1097/QAD.0000000000000783. AIDS. 2015. PMID: 26355573 Free PMC article.
-
Effect of antiretroviral therapy on clinical and immunologic disease progression in HIV positive children: One-year follow-up study.J Family Community Med. 2012 Sep;19(3):178-83. doi: 10.4103/2230-8229.102318. J Family Community Med. 2012. PMID: 23230384 Free PMC article.
References
-
- World Health Organization (WHO) AIDS epidemic update. WHO UNAIDS; 2007. Vol. UNAIDS/07.27E / JC1322E.
-
- Palumbo PE, Raskino C, Fiscus S, et al. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. JAMA. 1998;279:756–61. - PubMed
-
- Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. - PubMed
-
- Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–89. - PubMed