Dry powdered aerosols of diatrizoic acid nanoparticle agglomerates as a lung contrast agent
- PMID: 20214960
- PMCID: PMC2859999
- DOI: 10.1016/j.ijpharm.2010.03.009
Dry powdered aerosols of diatrizoic acid nanoparticle agglomerates as a lung contrast agent
Abstract
Aerosolized contrast agents may improve the resolution of biomedical imaging modalities and enable more accurate diagnosis of lung diseases. Many iodinated compounds, such as diatrizoic acid, have been shown to be safe and useful for radiographic examination of the airways. Formulations of such compounds must be improved in order to allow imaging of the smallest airways. Here, diatrizoic acid nanoparticle agglomerates were created by assembling nanoparticles into inhalable microparticles that may augment deposition in the lung periphery. Nanoparticle agglomerates were fully characterized and safety was determined in vivo. After dry powder insufflation to rats, no acute alveolar tissue damage was observed 2h post-dose. Diatrizoic acid nanoparticle agglomerates possess the characteristics of an efficient and safe inhalable lung contrast agent.
Copyright (c) 2010 Elsevier B.V. All rights reserved.
Figures

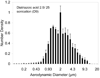





Similar articles
-
Effect of particle formulation on dry powder inhalation efficiency.Curr Pharm Des. 2010 Jul;16(21):2377-87. doi: 10.2174/138161210791920423. Curr Pharm Des. 2010. PMID: 20618158 Review.
-
Combination chemotherapeutic dry powder aerosols via controlled nanoparticle agglomeration.Pharm Res. 2009 Jul;26(7):1752-63. doi: 10.1007/s11095-009-9886-2. Epub 2009 May 5. Pharm Res. 2009. PMID: 19415471 Free PMC article.
-
Development and Evaluation of Chitosan Microparticles Based Dry Powder Inhalation Formulations of Rifampicin and Rifabutin.J Aerosol Med Pulm Drug Deliv. 2016 Apr;29(2):179-95. doi: 10.1089/jamp.2014.1187. Epub 2015 Sep 25. J Aerosol Med Pulm Drug Deliv. 2016. PMID: 26406162
-
Preparation and characterization of spray dried inhalable powders containing chitosan nanoparticles for pulmonary delivery of isoniazid.J Microencapsul. 2011;28(7):605-13. doi: 10.3109/02652048.2011.599437. Epub 2011 Jul 27. J Microencapsul. 2011. PMID: 21793647
-
Dry powder aerosol delivery systems: current and future research directions.J Aerosol Med. 2006 Spring;19(1):21-7. doi: 10.1089/jam.2006.19.21. J Aerosol Med. 2006. PMID: 16551211 Review.
Cited by
-
Nanoparticle agglomerates of fluticasone propionate in combination with albuterol sulfate as dry powder aerosols.Eur J Pharm Sci. 2011 Nov 20;44(4):522-33. doi: 10.1016/j.ejps.2011.09.014. Epub 2011 Sep 21. Eur J Pharm Sci. 2011. PMID: 21964203 Free PMC article.
-
Building membrane emulsification into pulmonary drug delivery and targeting.Pharm Res. 2010 Nov;27(11):2505-8. doi: 10.1007/s11095-010-0240-5. Epub 2010 Aug 12. Pharm Res. 2010. PMID: 20703894 No abstract available.
-
Rifampicin-Carbohydrate Spray-Dried Nanocomposite: A Futuristic Multiparticulate Platform For Pulmonary Delivery.Int J Nanomedicine. 2019 Nov 22;14:9089-9112. doi: 10.2147/IJN.S211182. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31819421 Free PMC article.
-
Iodinated NanoClusters as an inhaled computed tomography contrast agent for lung visualization.Mol Pharm. 2010 Aug 2;7(4):1274-82. doi: 10.1021/mp1000718. Mol Pharm. 2010. PMID: 20575527 Free PMC article.
-
Advances in device and formulation technologies for pulmonary drug delivery.AAPS PharmSciTech. 2014 Aug;15(4):882-97. doi: 10.1208/s12249-014-0114-y. Epub 2014 Apr 12. AAPS PharmSciTech. 2014. PMID: 24728868 Free PMC article. Review.
References
-
- Barrs TJ. Overview of radiopaque drugs. Am J Health Syst Pharm. 2006;63:2248–2255. - PubMed
-
- Bhavna, et al. Techniques to develop and characterize nanosized formulation for salbutamol sulfate. J Mater Sci Mater Med. 2009;20:71–76. - PubMed
-
- Bilati U, et al. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24:67–75. - PubMed
-
- Chan H-K, Chew NYK. Novel alternative methods for the delivery of drugs for the treatment of asthma. Advanced Drug Delivery Reviews. 2003;55:793–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

