Oseltamivir in seasonal influenza: cumulative experience in low- and high-risk patients
- PMID: 20215131
- PMCID: PMC2835508
- DOI: 10.1093/jac/dkq012
Oseltamivir in seasonal influenza: cumulative experience in low- and high-risk patients
Abstract
Seasonal influenza viruses cause annual disease epidemics that affect individuals at low and high risk for secondary illnesses. Influenza vaccines are widely used in high-risk patients to prevent infection, but the protection afforded varies by population; uptake is also limited in some groups. Antiviral drugs for influenza are now readily available. Oseltamivir is the most widely used antiviral for the treatment and prophylaxis of seasonal influenza, and its efficacy and safety are now well established in a variety of populations. In addition to decreasing the severity and duration of the symptoms of influenza, clinical and epidemiological studies demonstrate that oseltamivir significantly reduces the frequency of secondary illnesses and exacerbation of underlying conditions; survival is also significantly improved in seriously ill patients who are hospitalized with severe influenza. Resistant viruses are isolated with a low frequency during oseltamivir treatment (0.33% in adults and 4.0% in children among almost 2000 oseltamivir-treated patients enrolled onto Roche-sponsored clinical trials of oseltamivir treatment during the oseltamivir development programme). However, an oseltamivir-resistant influenza A (H1N1) virus emerged in Europe during the 2007-08 season and circulated in the southern and northern hemispheres in 2008-09. No link with oseltamivir usage could be detected, and the clinical impact of these viruses was limited. Oseltamivir-susceptible pandemic (H1N1) 2009 viruses now predominate in many countries. Oseltamivir is generally well tolerated, with a similar adverse event profile to placebo.
Figures
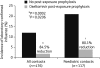


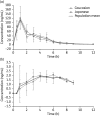

References
-
- CDC. Key Facts About Seasonal Influenza. http://www.cdc.gov/flu/keyfacts.htm. (22 October 2009, date last accessed)
-
- Ng TP, Pwee KH, Niti M, et al. Influenza in Singapore: assessing the burden of illness in the community. Ann Acad Med Singapore. 2002;31:182–8. - PubMed
-
- Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57:1–60. - PubMed
-
- Ruf BR, Szucs T. Reducing the burden of influenza-associated complications with antiviral therapy. Infection. 2009;37:186–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

