Oseltamivir in human avian influenza infection
- PMID: 20215132
- PMCID: PMC2835509
- DOI: 10.1093/jac/dkq013
Oseltamivir in human avian influenza infection
Abstract
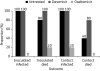
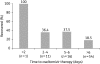
Avian influenza A viruses continue to cause disease outbreaks in humans, and extrapulmonary infection is characteristic. In vitro studies demonstrate the activity of oseltamivir against avian viruses of the H5, H7 and H9 subtypes. In animal models of lethal infection, oseltamivir treatment and prophylaxis limit viral replication and improve survival. Outcomes are influenced by the virulence of the viral strain, dosage regimen and treatment delay; it is also critical for the compound to act systemically. Observational data on oseltamivir treatment in the early stages of disease suggest it is useful for improving survival in patients infected with H5 viruses, and drug-selected resistance has only rarely been reported. The WHO strongly recommends oseltamivir for the treatment of confirmed or suspected cases of human H5 infection and prophylaxis of those at high risk of infection. In addition to oral dosing, nasogastric administration appears to be a viable option for the management of severely ill patients, as is the use of higher doses and prolonged schedules. F. Hoffmann-La Roche Ltd, the manufacturer of oseltamivir, is developing a mathematical model to allow rapid prediction of appropriate dosage regimens for any future pandemic. Roche is also funding the Avian Influenza Registry, an online database that aims to collect information from clinicians worldwide on the course of avian influenza in humans.
Figures
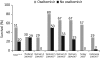
Similar articles
-
Responding to pandemic (H1N1) 2009 influenza: the role of oseltamivir.J Antimicrob Chemother. 2010 Apr;65 Suppl 2(Suppl 2):ii35-ii40. doi: 10.1093/jac/dkq014. J Antimicrob Chemother. 2010. PMID: 20215134 Free PMC article. Review.
-
Adherence with oseltamivir chemoprophylaxis among workers exposed to poultry during avian influenza outbreaks in southern Israel.Int J Infect Dis. 2009 Mar;13(2):261-5. doi: 10.1016/j.ijid.2008.06.037. Epub 2008 Oct 15. Int J Infect Dis. 2009. PMID: 18922718
-
The threat of an avian influenza pandemic.N Engl J Med. 2005 Jan 27;352(4):323-5. doi: 10.1056/NEJMp048343. Epub 2005 Jan 24. N Engl J Med. 2005. PMID: 15668220 No abstract available.
-
Infectious disease: can we avert a lethal flu pandemic?Curr Biol. 2005 Nov 22;15(22):R922-4. doi: 10.1016/j.cub.2005.10.063. Curr Biol. 2005. PMID: 16303549 Review.
-
Clinical and epidemiological characteristics of a patient infected with H5N6 avian influenza A virus.J Clin Virol. 2016 Sep;82:20-26. doi: 10.1016/j.jcv.2016.06.004. Epub 2016 Jun 14. J Clin Virol. 2016. PMID: 27395033 No abstract available.
Cited by
-
Insight into alternative approaches for control of avian influenza in poultry, with emphasis on highly pathogenic H5N1.Viruses. 2012 Nov 19;4(11):3179-208. doi: 10.3390/v4113179. Viruses. 2012. PMID: 23202521 Free PMC article. Review.
-
First human case of avian influenza A (H5N6) in Yunnan province, China.SAGE Open Med Case Rep. 2015 Aug 4;3:2050313X15596484. doi: 10.1177/2050313X15596484. eCollection 2015. SAGE Open Med Case Rep. 2015. PMID: 27489694 Free PMC article.
-
Nitraria schoberi L. hairy root culture as a source of compounds with antiviral activity against influenza virus subtypes А(H5N1) and А(H3N2).3 Biotech. 2018 Jun;8(6):260. doi: 10.1007/s13205-018-1280-5. Epub 2018 May 15. 3 Biotech. 2018. PMID: 29780682 Free PMC article.
-
Comparison of COVID-19 Health Risks With Other Viral Occupational Hazards.Int J Health Serv. 2021 Jan;51(1):37-49. doi: 10.1177/0020731420946590. Epub 2020 Aug 9. Int J Health Serv. 2021. PMID: 32772627 Free PMC article.
-
Antibodies Directed toward Neuraminidase N1 Control Disease in a Mouse Model of Influenza.J Virol. 2018 Jan 30;92(4):e01584-17. doi: 10.1128/JVI.01584-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29167342 Free PMC article.
References
-
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus. N Engl J Med. 2008;358:261–73. Update on avian influenza A (H5N1) virus infection in humans. - PubMed
-
- Lina B. [New pandemic influenza: what are the risks?] Rev Prat. 2008;58:1679–86. - PubMed
-
- Kandun IN, Wibisono H, Sedyaningsih ER, et al. Three Indonesian clusters of H5N1 virus infection in 2005. N Engl J Med. 2006;355:2186–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

