Sprouty2-modulated Kras signaling rescues Shp2 deficiency during lens and lacrimal gland development
- PMID: 20215346
- PMCID: PMC2835324
- DOI: 10.1242/dev.042820
Sprouty2-modulated Kras signaling rescues Shp2 deficiency during lens and lacrimal gland development
Abstract
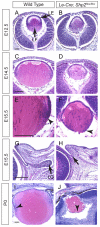
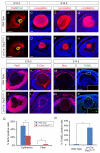
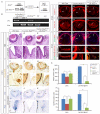
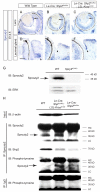
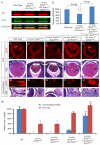
Shp2/Ptpn11 tyrosine phosphatase is a general regulator of the RTK pathways. By genetic ablation, we demonstrate that Shp2 is required for lacrimal gland budding, lens cell proliferation, survival and differentiation. Shp2 deletion disrupted ERK signaling and cell cycle regulation, which could be partially compensated by activated Kras signaling, confirming that Ras signaling was the main downstream target of Shp2 in lens and lacrimal gland development. We also showed that Sprouty2, a general suppressor of Ras signaling, was regulated by Shp2 positively at the transcriptional level and negatively at the post-translational level. Only in the absence of Sprouty2 could activated Kras signaling robustly rescue the lens proliferation and lacrimal-gland-budding defects in the Shp2 mutants. We propose that the dynamic regulation of Sprouty by Shp2 might be important not only for modulating Ras signaling in lens and lacrimal gland development, but also for RTK signaling in general.
Figures

Similar articles
-
Shc1 cooperates with Frs2 and Shp2 to recruit Grb2 in FGF-induced lens development.Elife. 2025 May 6;13:RP103615. doi: 10.7554/eLife.103615. Elife. 2025. PMID: 40327534 Free PMC article.
-
Frs2α and Shp2 signal independently of Gab to mediate FGF signaling in lens development.J Cell Sci. 2014 Feb 1;127(Pt 3):571-82. doi: 10.1242/jcs.134478. Epub 2013 Nov 27. J Cell Sci. 2014. PMID: 24284065 Free PMC article.
-
Sprouty-related Ena/vasodilator-stimulated phosphoprotein homology 1-domain-containing protein (SPRED1), a tyrosine-protein phosphatase non-receptor type 11 (SHP2) substrate in the Ras/extracellular signal-regulated kinase (ERK) pathway.J Biol Chem. 2011 Jul 1;286(26):23102-12. doi: 10.1074/jbc.M110.212662. Epub 2011 Apr 29. J Biol Chem. 2011. PMID: 21531714 Free PMC article.
-
Targeting KRAS and SHP2 signaling pathways for immunomodulation and improving treatment outcomes in solid tumors.Int Rev Cell Mol Biol. 2024;386:167-222. doi: 10.1016/bs.ircmb.2024.01.005. Epub 2024 Feb 20. Int Rev Cell Mol Biol. 2024. PMID: 38782499 Review.
-
[The Biological Function of SHP2 in Human Disease].Mol Biol (Mosk). 2016 Jan-Feb;50(1):27-33. doi: 10.7868/S0026898416010110. Mol Biol (Mosk). 2016. PMID: 27028808 Review. Russian.
Cited by
-
Deficient FGF signaling causes optic nerve dysgenesis and ocular coloboma.Development. 2013 Jul;140(13):2711-23. doi: 10.1242/dev.089987. Epub 2013 May 29. Development. 2013. PMID: 23720040 Free PMC article.
-
Understanding the role of growth factors in embryonic development: insights from the lens.Philos Trans R Soc Lond B Biol Sci. 2011 Apr 27;366(1568):1204-18. doi: 10.1098/rstb.2010.0339. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21402581 Free PMC article. Review.
-
Lacrimal gland budding requires PI3K-dependent suppression of EGF signaling.Sci Adv. 2021 Jun 30;7(27):eabf1068. doi: 10.1126/sciadv.abf1068. Print 2021 Jun. Sci Adv. 2021. PMID: 34193412 Free PMC article.
-
Feedback regulation of RTK signaling in development.Dev Biol. 2019 Mar 1;447(1):71-89. doi: 10.1016/j.ydbio.2017.10.017. Epub 2017 Oct 26. Dev Biol. 2019. PMID: 29079424 Free PMC article. Review.
-
A comprehensive review of SHP2 and its role in cancer.Cell Oncol (Dordr). 2022 Oct;45(5):729-753. doi: 10.1007/s13402-022-00698-1. Epub 2022 Sep 6. Cell Oncol (Dordr). 2022. PMID: 36066752 Review.
References
-
- Choi J., Park S. Y., Joo C. K. (2004). Hepatocyte growth factor induces proliferation of lens epithelial cells through activation of ERK1/2 and JNK/SAPK. Invest. Ophthalmol. Vis. Sci. 45, 2696-2704 - PubMed
-
- Cunnick J. M., Meng S., Ren Y., Desponts C., Wang H. G., Djeu J. Y., Wu J. (2002). Regulation of the mitogen-activated protein kinase signaling pathway by SHP2. J. Biol. Chem. 277, 9498-9504 - PubMed
-
- Dance M., Montagner A., Salles J. P., Yart A., Raynal P. (2008). The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell. Signal. 20, 453-459 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

